Sponsored content brought to you by
Over the years, cancer researchers have tried to characterize the complex network of protein and cellular interactions that support or inhibit tumor growth. Spatial context provides a new perspective that is rapidly improving scientists’ understanding of how cancers progress.
Immunohistochemistry (IHC) assays have long been the standard for imaging and analyzing tissue samples. What IHC cannot provide is multiplexing scale: the ability to look at many complex cellular interactions within the tumor microenvironment at once. The result is a less-than-comprehensive view of the biology of tumors, which has until recently limited the development of new therapies for cancer.
MIBI-enabled spatial proteomics provides more detailed insights into the tumor microenvironment
Ionpath’s high-resolution multiplexed ion beam imaging (MIBI) spatial proteomics technology provides key information—not previously available with IHC—that cancer researchers are utilizing to unravel new details about the tumor microenvironment in several cancers.1,2,3 With MIBI technology, researchers can confidently identify, enumerate, and map different cell populations based on specific markers. They can also quantify and map expression of key protein biomarkers (e.g., immune checkpoints) with spatial context and assess immune infiltration in tumor samples.
MIBI’s unique advantage for tissue analysis is due to its superior performance in several areas: high multiplexing capacity, high image resolution, and high signal detection sensitivity while maintaining compatibility with FFPE tissue samples and common histology workflows.
Researchers probe the information rich-cellular landscape of tumor and disease microenvironments with MIBI by leveraging 40+ markers simultaneously to deliver comprehensive datasets and novel biological insights.
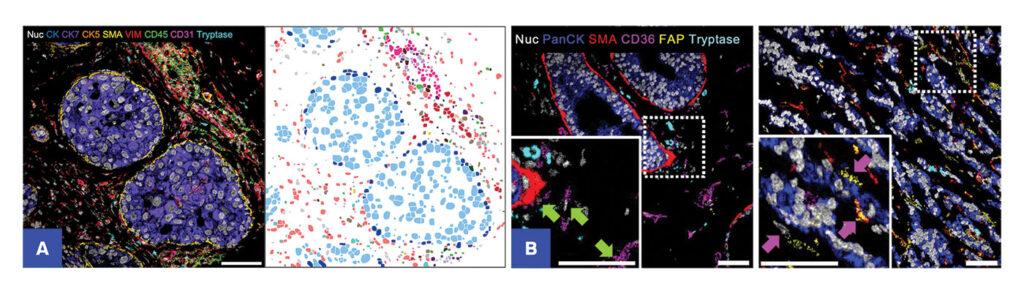
MIBI provides novel insights into cancer biology
The high-resolution, spatial, and quantitative information that MIBI provides is enabling the discovery of spatial signatures in cancer that can inform disease progression and guide therapy selection, discovery of immunosuppressive cell populations within tumor landscapes, and cell populations linked to metastasis and poor prognostic outcomes.
In a recent breast cancer study1 the transition from ductal carcinoma in situ (DCIS) to invasive breast cancer (IBC), which occurs in up to half the patients diagnosed with DCIS, was studied using MIBI. Scientists at Stanford and other institutions characterized the tumor-immune and stroma landscape in a longitudinal patient cohort and revealed spatial predictors of cancer progression. Ultimately, findings like this increase our understanding of disease progression and bridge gaps in our ability to accurately predict which patients will progress to invasive forms of cancer and prevent non-progressing patients from undergoing unnecessary medical procedures.
In another study, researchers at Memorial Sloan Kettering Cancer Center and their collaborators combined MIBI-enabled spatial proteomics with single-cell transcriptomic data from small cell lung cancer cases to better understand the factors involved in metastasis and poor prognostic outcomes.2 With MIBI technology, scientists identified cell populations that contributed to immunosuppression and increased metastasis.
And in a third example, a team at the University of Minnesota used MIBI spatial proteomics to characterize immune cell infiltrates in the tumor microenvironments of certain aggressive forms of breast cancer.3 MIBI analysis provided rich spatial and quantitative detail that enabled them to discover clues of immune cell interactions in solid tumors and their local interactions in distinct tumor regions.
MIBI-enabled spatial proteomics has the potential to elucidate tumor biology in new ways. MIBI has already transformed discovery and translational cancer research by providing scientists with a tool to increase the understanding of cancer and identify new spatial signatures of disease progression that will catalyze the next generation of treatment strategies.
Ionpath offers opportunities to experience the high-quality imaging data and MIBI analysis through its Spatial Proteomic Services or the purchase of a MIBIscope™ system.
References
- Risom, T., Glass, D. R., et al. Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma. Cell, 185(2), 299–3 10.e18. doi.org/10.1016/j.cell.2021.12.023, 2022.
- Chan, J. M., Quintanal-Villalonga, Á., et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell, 39(11), 1479–1496.e18. doi.org/10.1016/j.ccell.2021.09.008, 2021.
- He, Y., Barthel, G.M., et al. [SITC 22 Poster #1446] Multiplexed ion beam imaging identifies B-cell enrichment in the RHAMM-high invasive niche of breast cancer, 2022.
To learn more about how MIBI can accelerate your research, visit ionpath.com/personalized-insights-into-cancer or talk with us at Booth #515 at AACR 2023.











