Sponsored content brought to you by
Precise diagnostics can trigger cancer revolution, either by detecting cancer earlier, when still curable, or by identifying the right patient for a specific therapy, with the goal to save lives, improve quality of life, and better manage health care costs.1
For precise diagnostics precise protocols are required, starting from sample handling up to sample analysis: the pre-analytical step is not only a mere technological issue but a real clinical need, since a wrong diagnosis can arise just from an unstable sample.
Tethis has therefore focused its research and development effort in sample preparation and pre-analytical standardization, to be able to collect and make available rare, unstable, and heterogeneous tumor biomarkers from blood samples.
Pre-analytical workflow for an enrichment-free assay
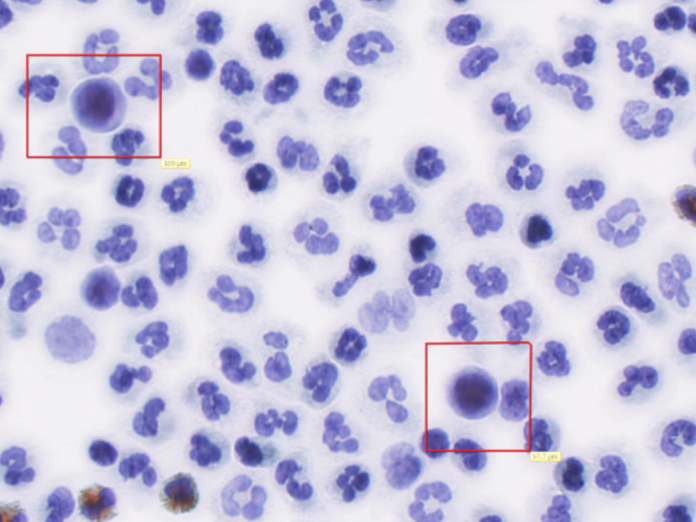
 The pre-analytical flow is based on the utilization of fresh blood collected in EDTA without preservatives that will be processed, within few hours, by See.d, placed at the point of blood collection; the platform utilizes the proprietary SBS slide that collects and immobilizes the whole WBCs fraction, including rare and heterogeneous circulating tumor cells (CTCs) eventually deriving from the tumor itself.
The pre-analytical flow is based on the utilization of fresh blood collected in EDTA without preservatives that will be processed, within few hours, by See.d, placed at the point of blood collection; the platform utilizes the proprietary SBS slide that collects and immobilizes the whole WBCs fraction, including rare and heterogeneous circulating tumor cells (CTCs) eventually deriving from the tumor itself.
SBS slides allow their immediate and spontaneous adhesion at room temperature, allowing shear stress-free collection of the entire WBC fraction; in these conditions there is no cellular selection and consequently no bias toward a specific cellular component; the feature of this slide allows a uniform distribution of cells in monolayer resembling a 2D tissue slide, available for multiple downstream analyses.
All relevant content for liquid biopsy available for analysis
 See.d performs an automated, standardized and gentle preparation of a blood sample at the point of blood collection, as a stand-alone platform. Sample is stabilized on SBS slide for CTCs detection while plasma from the same sample is made available for the analysis of cell-free content. See.d does not require specialized lab technicians and most importantly there is no need of blood shipment, one of the most critical points of pre-analitycs in liquid biopsy. See.d processes fresh blood sample in EDTA shortly after collection (within 2 hours). All sample preparation steps are automated: blood separation between cellular fraction and plasma by centrifugation, red blood cell lysis to collect WBCs, cell seeding and fixing on SBS slides and plasma recovery in tubes. At the end of the process, up to 20 fixed slides/case can be provided, and the relative plasma is collected in a separate tube. Specimens are both stable and ready to be shipped for downstream analysis that will allow multi-analytes evaluation starting from a fresh blood sample.
See.d performs an automated, standardized and gentle preparation of a blood sample at the point of blood collection, as a stand-alone platform. Sample is stabilized on SBS slide for CTCs detection while plasma from the same sample is made available for the analysis of cell-free content. See.d does not require specialized lab technicians and most importantly there is no need of blood shipment, one of the most critical points of pre-analitycs in liquid biopsy. See.d processes fresh blood sample in EDTA shortly after collection (within 2 hours). All sample preparation steps are automated: blood separation between cellular fraction and plasma by centrifugation, red blood cell lysis to collect WBCs, cell seeding and fixing on SBS slides and plasma recovery in tubes. At the end of the process, up to 20 fixed slides/case can be provided, and the relative plasma is collected in a separate tube. Specimens are both stable and ready to be shipped for downstream analysis that will allow multi-analytes evaluation starting from a fresh blood sample.
SBS slides are compatible with all immunostaining technologies from brightfield to immunofluorescence, FISH up to multiplexing; thanks to AI-supported imaging. we are establishing novel algorithms for efficient identification of tumor cells with different biomarkers. SBS slides are also suitable for single cell molecular analysis thanks to single cell microdissection.
Pilot study on early breast cancer detection
A first POC of the See.d prototype has been recently published in BJC,2 a work done in collaboration with Basel University in early breast cancer, that confirms the validity of the pre-analytical platform for identification of CTCs as single cells and CTC clusters, demonstrating the capability of providing the heterogeneity of the tumor biomarkers present in blood, even at early stages, and opening new perspectives for the exploitation of this technology in different clinical contexts, thanks to the precise definition of all the critical pre-analytical requirements to provide a fully informative clinical sample.
References
1. Brown, Noah A, and Kojo S J Elenitoba-Johnson. “Enabling Precision Oncology Through Precision Diagnostics.” Annual review of pathology vol. 15 (2020): 97-121. doi:10.1146/annurev-pathmechdis-012418-012735
2. Krol, Ilona et al. “Detection of clustered circulating tumour cells in early breast cancer.” British journal of cancer vol. 125,1 (2021): 23-27. doi:10.1038/s41416-021-01327-8
For more information: www.tethis-lab.com, [email protected]












