Decades ago, the oncology community took on the crucial challenge of detecting cancer at the earliest possible stage. Overall, survival rates improve dramatically for cancers detected before metastasis. For example, the U.S. Centers for Disease Control and Prevention (CDC) notes that the five-year survival rate for breast cancer patients more than triples if the disease is diagnosed when it’s still local to the breasts, compared with a diagnosis after the cancer has spread to distant tissues.1 Despite today’s detection technology, however, many cancers still go undetected until after the disease has spread. Instead of detecting cancers one by one, various technologies promise multi-cancer early detection (MCED), and some of these approaches are already being used.

CSO
The Cancer Prevention and Research Institute of Texas
“Cancer screening works by reducing the risk of death and morbidity through the detection of well-defined and clinically important precancerous or early invasive lesions, which are more amenable to curative treatments than when detected from clinical presentation,” says Michelle Le Beau, PhD, chief scientific officer of the Cancer Prevention and Research Institute of Texas (CPRIT). “Routine population-based screening is currently recommended only for breast, cervical, colorectal, and lung cancers, which are relatively common and have evidence of benefits related to reductions in the risk of death that outweigh the harms for the respective screening tests.”
Patients could gain huge benefits from MCED. According to a panel study conducted by Lee Schwartzberg, MD, chief of medical oncology and hematology at the William N. Pennington Cancer Institute in Reno, Nevada, and his colleagues: “Experts rated 85% (n = 17) of cancers as somewhat likely to extremely likely to be cured in stage I, 60% (n = 12) in stage II, 5% (n = 1) in stage III, 0% in stage IV.”2 Those opinions reveal the stark outcomes of cancer that is discovered late, often too late.
In addition to saving more lives, MCED could save money spent on healthcare. Allan Hackshaw, PhD, director of the UCL (University College London) Cancer Trials Centre and Cancer Research UK, and his colleagues estimated that current methods of screening a patient for breast, cervical, colorectal, and lung cancer could cost nearly $90,000, but MCED could do all of that screening in one go for about $7,000—making it nearly 13 times less expensive.3 Such healthcare savings quickly grow into gigantic financial returns.
To reach those clinical and economic benefits, oncologists need MCED technology that is easy and accurate. A collection of ongoing approaches might eventually make late-stage cancer diagnosis a thing of the past—for humans and even some of their pets. (See “Catching Cancer Sooner in Dogs.”)
Methods of MCED tests
Rather than analyzing a tissue or scanning a patient, MCED tests “encompass a range of technologies that target multiple cancers using samples including blood, breath, urine, saliva, or stool,” Le Beau explains. “When added to existing approaches to single-cancer screening, these emerging technologies could provide clinicians with the opportunity to identify a broad range of cancers earlier in the course of the disease, raising the potential to treat them more effectively.”
Crucially, scientists and companies are developing MCED tests for patients with no sign of cancer. To accomplish that, an MCED test looks for cancer-based biomarkers, such as DNA mutations or methyl marks, that are representative of tumor cells. “While all MCED tests indicate if a cancer signal is present, some provide molecular information about the likely organ of origin,” Le Beau notes. “When an initial signal is positive for cancer, further analysis can be conducted to determine the source of the cancer, which may provide clinicians with information for follow-up testing for a confirmed diagnosis.”
Although many MCED tests are going through various stages of research and development, none have been approved by the U.S. Food and Drug Administration (FDA). Nonetheless, the FDA has granted Breakthrough Device designation to a few.

SVP of medical affairs and CMO
GRAIL
One of those is Galleri from GRAIL, which was acquired by California-based Illumina in 2021. Galleri “can identify a common cancer signal shared by more than 50 types of cancer,” says Jeff Venstrom, MD, senior vice president of medical affairs and chief medical officer at GRAIL. This test relies on next-generation sequencing (NGS) of a blood sample, followed by analysis based on machine learning—all of which takes about 10 days to complete. Although MCED might reduce some of the costs of some cancer testing, this acquisition suggested that reaching such economic goals could be expensive—Illumina paid $7.1 billion for GRAIL.
Another example is CancerSEEK, which came from work by Bert Vogelstein, MD, professor of oncology at Johns Hopkins Medicine, and his colleagues.4 These scientists built this test around their own NGS technology, called Safe-SeqS, now owned by Illumina. Development of this test for commercial use started with Boston-based Thrive Earlier Detection, which was acquired by Exact Sciences in 2020. Compared to the GRAIL purchase, Exact Sciences paid far less, only $2.15 billion, for Thrive. Still, that’s a significant investment, especially in an industry where no MCED test has been approved by the FDA so far.
Recently, Burning Rock, which is headquartered in China, received Breakthrough Device designation for its OverC Multi-Cancer Detection Blood Test. This test relies on methylation of cell-free DNA (cfDNA) to detect signs of esophageal, liver, lung, ovarian, and pancreatic cancers. Early in 2022, this test received the European Union’s Conformité Européenne (CE) Mark, allowing it to be sold in EU countries.
Sequencing cell-free DNA
A person’s blood always contains cfDNA, but it’s usually just from blood cells. In someone with a tumor, though, the blood can also include circulating tumor DNA (ctDNA). As Samantha Hasenleithner, PhD, and Michael Speicher, MD, both at the Diagnostic and Research Institute of Human Genetics at the Medical University of Graz in Austria, wrote: “Multiple studies have demonstrated the value of ctDNA analyses at various stages throughout the clinical course.”5

senior scientist
Diagnostic and Research Institute of Human Genetics, Medical University of Graz
Nonetheless, ctDNA is a challenging biomarker. First, there’s not much of it, which makes detection difficult. Plus, the heterogeneity of ctDNA creates another challenge. “Different types of cancer and even different tumors within the same type of cancer can have different genomic alterations,” Hasenleithner says. “Therefore, it can be challenging to develop a single blood test that can detect all types of cancer.” Accuracy can also be problematic. Someone without cancer can have altered cfDNA, which might be misidentified ctDNA, and there can be no detectable ctDNA in someone with cancer.
Machine learning can help scientists analyze cfDNA. As Hasenleithner explains: “Machine learning can be used to develop algorithms that can analyze large amounts of genomic data and identify patterns that are associated with specific types of cancer, enabling the improvement of the accuracy and reliability of cfDNA-based tests.” Consequently, “machine learning algorithms can help to distinguish between healthy individuals and patients with cancer,” she says. (For more on this topic, see the recent review by Ellen Heitzer, PhD, of the Medical University of Graz and her colleagues.6)
Beyond just identifying cfDNA, scientists can explore its features. For example, “cfDNA methylation can provide information on the tissue of origin of the cancer, which can be useful for early cancer detection and diagnosis,” Hasenleithner notes. As one example, Galleri detects methylation patterns in cfDNA. In addition, Hasenleithner and Speicher reported on the potential of using open chromatin regions, such as starting sites for transcription, as an indicator of cancer.7
This field is growing increasingly complex, especially as these new technologies inform precision-oncology approaches. For this reason, Speicher and Hasenleithner have begun an initiative called Vessel, which they say seeks to bridge the gap between NGS-based technologies and clinical application.8

Questions to consider
Despite the appeal of MCED and the large investments in the technology, many questions remain. Le Beau provides a long list. At the top of this list, she asks: “What percentage of patients with a positive MCED screening test actually have cancer?” That is a key question. Although the answer varies between MCED tests, Venstrom says that Galleri has a “false-positive rate of less than 1%.”
In terms of identifying a cancer and its source, some MCED tests produce impressive results. In GRAIL’s PATHFINDER study, for example, “Adding MCED screening to standard of care screening more than doubled the number of cancers detected,” Venstrom notes, and the “MCED-predicted cancer signal origin had 97.1% accuracy.”
Even if MCED tests detect cancer accurately and identify the type of cancer, other questions remain. For instance, Le Beau asks: “Will early screening with these tests lead to early diagnosis and more curable stages of cancer?” Even if the answer turns out to be yes, Le Beau points out another concern: “Will MCED testing and associated diagnostic tests be cost-effective and will they be accessible to all populations?” Even though some studies suggest that MCED testing might be less expensive than conventional methods, the big spending going on in this section of oncology research and development raises questions about the overall economy that will surround the MCED sector.
One additional fundamental question that must be answered is: When would MCED tests be used? In fact, this general question spawns a collection of related concerns, such as: Should patients be retested? If so, how often and based on what criteria? Like many new technologies in healthcare, the questions can outstrip the answers, and MCED testing is not immune to that challenge. Providing answers to these crucial questions about MCED testing will take more time, research, and investment.
Targeting transposons
Despite the recent rush of research and development around MCED testing, some of the work arises from a deeper history, and sometimes an unexpected one. In the 1940s and ’50s, for example, the late cytogeneticist Barbara McClintock showed that some of the DNA sequences in corn can move around in the plant’s genome. The discovery of these transposons, sometimes called jumping genes, earned McClintock the 1983 Nobel Prize in Physiology or Medicine, but this phenomenon goes beyond agricultural fields.
“It’s been known for 30 years that transposons could be involved in cancer in some way,” says Martin Taylor, MD, PhD, instructor in pathology at Harvard Medical School in Boston. “In the early 1990s, Bert Vogelstein showed that insertional mutagenesis could disrupt cancer genes.”
While studying how transposons work, Taylor and his colleagues developed antibodies that target proteins from one of those transposons, known as long interspersed nuclear element 1 (LINE-1). About a decade ago, Taylor and Kathleen Burns, MD, PhD, now chairperson of pathology at the Dana-Farber Cancer Institute at Harvard Medical School in Boston, put those antibodies on tissue microarrays. “We were really shocked to find that, across carcinomas, the rates of positivity were very, very high,” Taylor says. “Across most major carcinoma types in our initial study, the rates of finding overexpression in LINE-1 transposon were between 50 and 95 percent or higher.”
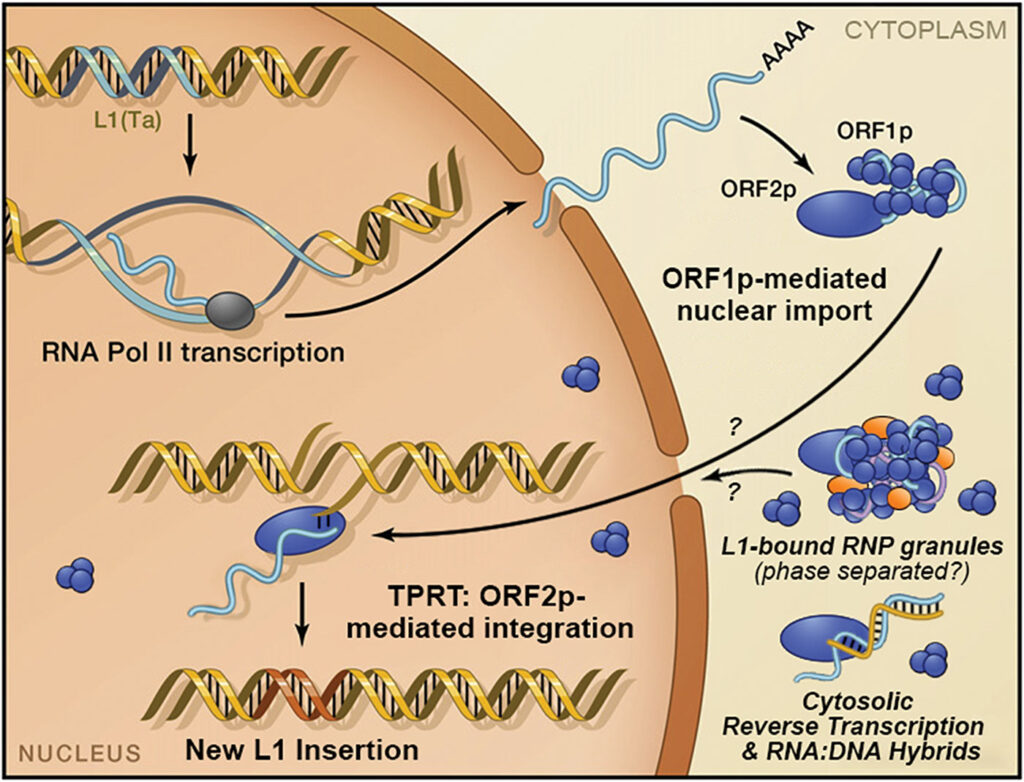
As Taylor explains: “From many sequencing efforts over the past 10 years, we now know that LINE insertions are ubiquitous in cancer genomes, especially in carcinomas.” Plus, such LINE insertions appear early in cancer. Instead of being a byproduct of cancer, these LINE insertions might play a role in the development of disease.
Through various collaborations, Taylor and his colleagues kept improving the sensitivity of finding LINE-1 proteins. “We built up a whole bunch of affinity reagents that enabled us to find these proteins at the incredibly low circulating concentrations that are typical in cancer patients,” he says. From running larger and larger studies on people with and without cancer, Taylor says, it was “shocking that the healthy people were predominantly negative, and when we looked deeper at the people that had signaled the presence of LINE-1 proteins, there was often something going on, some premalignant state—or they actually had cancer that had not yet been detected.”
Recently, Taylor, Burns, and their colleagues reported on LINE-1 open reading frame 1 protein (ORF1p).9 With digital immunoassays, the scientists could detect as few as about 1,000 single molecules of ORF1p in plasma, and they found that this biomarker could be a very specific diagnostic tool for a range of cancers. From this study, the research team concluded: “Plasma ORF1p shows promise for early detection of ovarian cancer, improves diagnostic performance in a multi-analyte panel, and provides early therapeutic response monitoring in gastric and esophageal cancers.”
Beyond being specific, an ORF1p-targeted test could provide other benefits. “We can use as little as 25 microliters of plasma—at least for the published studies—and the results can be turned around in a couple hours,” Taylor says. “So, you can imagine running this as a routine test in the hospital or in the lab.” Taylor and colleagues are exploring these as both standalone tests and as part of MCED tests.
Teaming up to improve MCED
Much like MCED combines multiple cancer tests in one, teams of experts work together to advance this technology. One of those is the MCED Consortium, which is a U.S./U.K. nonprofit consortium. Its mission is “to evaluate emerging data from MCED studies and establish standards in MCED technology by defining the clinical and public-health value of the technology, providing guidance for its use in clinical practice, and developing a public-outreach approach that identifies and mitigates potential health inequities that could arise from the use of MCED technology.”
As the chair of the MCED Consortium, Le Beau describes its wide range of participants, including experts in oncology, epidemiology, genomics, behavioral science, primary care, and patient advocacy. Although this consortium approaches MCED testing from a broad perspective, one objective that Le Beau highlights is “defining and reaching broad consensus on how to evaluate MCED tests in the clinical context and create processes for creating a clinical utility framework.”
It’s not just nonprofit organizations that create teams to assess and advance MCED testing. One such example comes from the U.K.’s National Health Service (NHS) and GRAIL. These groups are teaming up to run a randomized clinical trial to assess the use of Galleri for NHS’s cancer screening. According to Venstrom: “GRAIL completed enrollment of more than 140,000 individuals, and year two follow-up testing has commenced.”
The U.K.’s NHS is not the only government organization working on a large study of MCED testing. The U.S. National Cancer Institute (NCI) will be running its Vanguard Study, which is part of President Joe Biden’s Cancer Moonshot initiative. This clinical trial will start enrolling participants in 2024. A White House fact sheet describes Vanguard as “a large national trial that, if successful will identify effective blood tests for the detection of one or more cancers, providing the opportunity for additional, less-invasive tools for early detection.”10
In sum, researchers push on to find new indicators of cancer and better ways to assess them, companies invest large amounts of money in developing MCED tests, governments set up clinical trials, and groups collaborate to investigate the status of technologies and trial results Still, the oncology community must wait for the ultimate answers to questions from patients. An early cancer diagnosis, even one far earlier than is currently possible, does not benefit a patient unless this information produces an answer to the ultimate question: Will I survive?
The answer to that question could be years away. As Le Beau concludes: “The greatest challenge is that it may take more than 10 years to assess the effect on mortality, and it will be critical to consider evaluating intermediate endpoints, such as an increase in cases diagnosed at an earlier stage with a concomitant decrease in the number of advanced stage cases.”
References
- U.S. Centers for Disease Control and Prevention. Incidence and relative survival by stage at diagnosis for common cancers (Last reviewed November 10, 2021.).
- Schwartzberg, L., Broder, M.S., Ailawadhi, S., et al. Impact of early detection on cancer curability: A modified Delphi panel study. PLOS ONE 17(12): e0279227 (2022).
- Hackshaw, A., Cohen, S.S., Reichert, H., et al. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. British Journal of Cancer 125:1432–1442 (2021).
- Cohen, J.D., Li, L., Wang, Y., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359(6378):926–930 (2018).
- Hasenleithner, S.O., Speicher, M.R. How to detect cancer early using cell-free DNA. Cancer Cell 40:1464–1466 (2022).
- Moser, T., Kühberger, S., Lazzeri, I., et al. Bridging biological cfDNA and machine learning approaches. Trends in Genetics DOI:10.1016/j.tig.2023.01.004 (2023).
- Hasenleithner, S.O., Speicher, M.R. A clinician’s handbook for using ctDNA throughout the patient journey. Molecular Cancer 21:81 (2022).
- Vessel.
- Taylor, M.S., Wu, C., Fridy, P.C., et al. Ultrasensitive detection of circulating LINE-1 ORF1p as a specific multi-cancer biomarker. bioRxiv DOI:10.1101/2023.01.25.525462 (2023).
- The White House. FACT SHEET: President Biden Details Cancer Moonshot Progress and New Initiatives on 60th Anniversary of President Kennedy’s Moonshot Address (2022).
- American Medical Veterinary Association. Cancer in pets.










