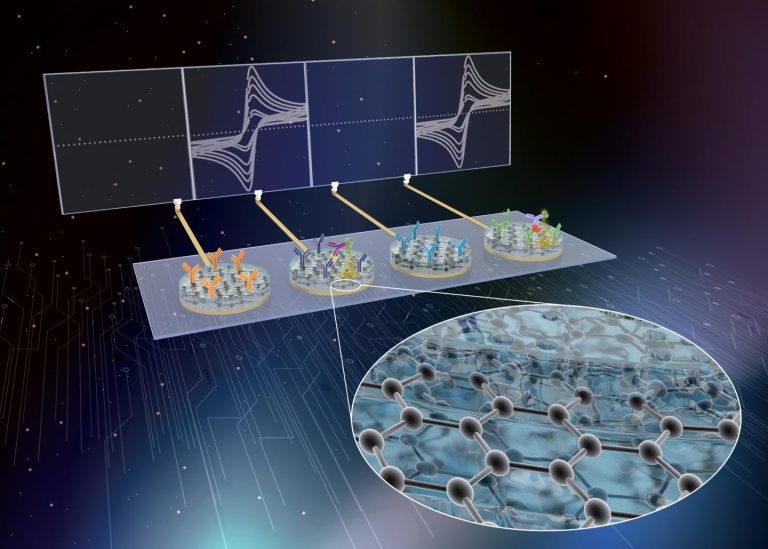
A specialized biosensor allows quick and accurate detection of multiple sepsis biomarkers – procalcitonin, C-reactive protein and pathogen-associated molecular patterns — from a blood sample.
The sensor was developed as a collaboration between Harvard’s Wyss Institute for Biologically Inspired Engineering and the University of Bath in the UK. It combines graphene nanoparticles with a bovine serum albumin composite to allow the detection of multiple biomarkers in one test.
Sepsis is an inflammatory immune response that is triggered in response to an infection. It can be life threatening and patients often deteriorate fast. This means that every minute counts and a quick diagnosis is important to make sure patients are treated correctly and have the best chance of a fast recovery.
Various attempts have been made to produce fast, point of care electrochemical tests for different biomarkers, but so far these all test one biomarker at a time. Some, such as Abbott’s i‐STAT system, allow the use of different cartridges for different biomarkers, but they cannot test concurrently for more than one biomarker.
A confirmed sepsis diagnosis requires testing of multiple different biomarkers, but running several separate tests takes time. It has therefore long been a goal to develop an electrochemical sensor that can run multiple tests at the same time.
Wyss Founding Director Donald Ingber, M.D., Ph.D., and colleagues achieved this by combining graphene nanoparticles, which helps prevent biological material sticking to the sensor while maintaining electroconductivity, with a thin composite coating of bovine serum albumin. When desired biomarkers stick to the coating, an electrical signal is generated and a measurement recorded.
This is an advance on previous technology developed by the same group that used gold rather than graphene. “We replaced the coating’s gold nanowires with graphene oxide nanoflakes that also have anti-fouling and electrochemical properties, but they are much less expensive and allow even more sensitive measurements. In fact, the costs of fabricating the nanocomposite were reduced to a fraction of its original cost,” said Wyss Senior Staff Scientist Pawan Jolly, Ph.D., who also worked on the technology.
The team first added antibodies to the sensor that could detect procalcitonin, which is produced by many cells in response to bacterial infections and checked its accuracy compared with a standard ELISA assay.
They then created a multiplex sensor by adding elements that could detect C-reactive protein, a marker of inflammation often raised in sepsis, and pathogen-associated molecular patterns. The latter element uses a genetically modified protein called FcMBL, developed at Wyss, that has the ability to bind over 100 different pathogenic microbes and their associated biomarkers, which are often released into the blood during sepsis.
As reported in the journal Advanced Functional Materials, the researchers found that the multiplex sensor detected biomarkers in a clinically significant range and did not show any cross-reactivity, which has caused problems with accuracy in the past.
“Assembling three dedicated electrochemical sensor elements for biomarkers that can be present in blood at vastly different concentrations on a single chip posed a significant challenge. However, the three elements in the final sensor exhibited specific responses within the clinically significant range without interfering with each other, and they did so with a turnaround time of 51 minutes, which meets the clinical need of sepsis diagnosis within the first hour,” said Uroš Zupančič, a Ph.D. student who was previously a visiting scholar in Ingber’s group from the University of Bath.
To create a proof of concept, the team integrated the procalcitonin sensor with a microfluidic system that allows inclusion of more biomarker binding sites and adds automation. This allowed detection within 7 minutes. Although only one biomarker was tested in this way, the researchers think it should be fairly straightforward to add additional sensors in close proximity on the same chip.
“When coupled with microfluidics, these novel multiplexed sensors could detect a panel of biomarkers in whole blood in a matter of minutes, opening the way to low‐cost and easy‐to‐use rapid sepsis diagnostics,” conclude the authors.
A specialized biosensor allows quick and accurate detection of multiple sepsis biomarkers – procalcitonin, C-reactive protein and pathogen-associated molecular patterns — from a blood sample.
The sensor was developed as a collaboration between Harvard’s Wyss Institute for Biologically Inspired Engineering and the University of Bath in the UK. It combines graphene nanoparticles with a bovine serum albumin composite to allow the detection of multiple biomarkers in one test.
Sepsis is an inflammatory immune response that is triggered in response to an infection. It can be life threatening and patients often deteriorate fast. This means that every minute counts and a quick diagnosis is important to make sure patients are treated correctly and have the best chance of a fast recovery.
Various attempts have been made to produce fast, point of care electrochemical tests for different biomarkers, but so far these all test one biomarker at a time. Some, such as Abbott’s i‐STAT system, allow the use of different cartridges for different biomarkers, but they cannot test concurrently for more than one biomarker.
A confirmed sepsis diagnosis requires testing of multiple different biomarkers, but running several separate tests takes time. It has therefore long been a goal to develop an electrochemical sensor that can run multiple tests at the same time.
Wyss Founding Director Donald Ingber, M.D., Ph.D., and colleagues achieved this by combining graphene nanoparticles, which helps prevent biological material sticking to the sensor while maintaining electroconductivity, with a thin composite coating of bovine serum albumin. When desired biomarkers stick to the coating, an electrical signal is generated and a measurement recorded.
This is an advance on previous technology developed by the same group that used gold rather than graphene. “We replaced the coating’s gold nanowires with graphene oxide nanoflakes that also have anti-fouling and electrochemical properties, but they are much less expensive and allow even more sensitive measurements. In fact, the costs of fabricating the nanocomposite were reduced to a fraction of its original cost,” said Wyss Senior Staff Scientist Pawan Jolly, Ph.D., who also worked on the technology.
The team first added antibodies to the sensor that could detect procalcitonin, which is produced by many cells in response to bacterial infections and checked its accuracy compared with a standard ELISA assay.
They then created a multiplex sensor by adding elements that could detect C-reactive protein, a marker of inflammation often raised in sepsis, and pathogen-associated molecular patterns. The latter element uses a genetically modified protein called FcMBL, developed at Wyss, that has the ability to bind over 100 different pathogenic microbes and their associated biomarkers, which are often released into the blood during sepsis.
As reported in the journal Advanced Functional Materials, the researchers found that the multiplex sensor detected biomarkers in a clinically significant range and did not show any cross-reactivity, which has caused problems with accuracy in the past.
“Assembling three dedicated electrochemical sensor elements for biomarkers that can be present in blood at vastly different concentrations on a single chip posed a significant challenge. However, the three elements in the final sensor exhibited specific responses within the clinically significant range without interfering with each other, and they did so with a turnaround time of 51 minutes, which meets the clinical need of sepsis diagnosis within the first hour,” said Uroš Zupančič, a Ph.D. student who was previously a visiting scholar in Ingber’s group from the University of Bath.
To create a proof of concept, the team integrated the procalcitonin sensor with a microfluidic system that allows inclusion of more biomarker binding sites and adds automation. This allowed detection within 7 minutes. Although only one biomarker was tested in this way, the researchers think it should be fairly straightforward to add additional sensors in close proximity on the same chip.
“When coupled with microfluidics, these novel multiplexed sensors could detect a panel of biomarkers in whole blood in a matter of minutes, opening the way to low‐cost and easy‐to‐use rapid sepsis diagnostics,” conclude the authors.