A new study details how some SARS-CoV-2 variants escape immune defense, in either vaccinated or unvaccinated people. The researchers used structural biology to map, at atomic scale, how certain classes of neutralizing antibodies bind to the original pandemic strain of SARS-CoV-2, the virus that causes COVID-19. They also showed how that process is disrupted by mutations found in new variants that were first detected in Brazil, the United Kingdom, South Africa and India.
The scientists, whose study appears in Science this week, are from Scripps Research along with collaborators in Germany and the Netherlands.
According to their research, several of these mutations are clustered in the receptor binding site on the virus’s spike protein. Other sites on that domain are unaffected.
“An implication of this study is that, in designing next-generation vaccines and antibody therapies, we should consider increasing the focus on other vulnerable sites on the virus that tend not to be affected by the mutations found in variants of concern,” says co-lead author Meng Yuan, Ph.D., a postdoctoral research associate in the laboratory of senior author Ian Wilson, D.Phil, a professor at Scripps Research.
Because of their potential to spread diseases, even despite vaccination, there is a of attention on these variants, which include the UK’s B.1.1.7, South Africa’s B.1.351, Brazil’s P.1, and India’s B.1.617. Some of these variants appear to be more infectious than the original Wuhan strain. Further, studies have found that antibody responses generated either by natural infection by the original strain or via vaccination are less effective in neutralizing these strains.
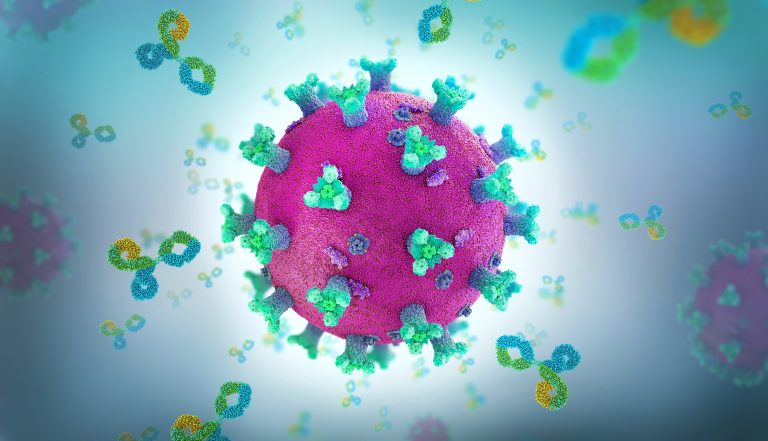
These researchers focused mainly on three mutations in the SARS-CoV-2 spike protein: K417N, E484K, and N501Y. Alone or in combination, these mutations are found in most major SARS-CoV-2 variants. All of the mutations are found in the SARS-CoV-2 receptor binding site, which the where the virus attaches to host cells. The team tested antibodies from the major classes that target the area in and around the receptor binding site and found that many lose their ability to effectively bind and neutralize the virus when the mutations are present.
Using structural imaging techniques, the team then mapped the relevant portion of the virus at atomic-scale resolution to examine how the mutations affect sites where antibodies otherwise would bind and neutralize the virus.
“This work provides a structural explanation for why antibodies elicited by COVID-19 vaccines or natural infection by the original pandemic strain are often ineffective against these variants of concern,” Wilson says.
The findings suggest that while antibody responses can be very potent in neutralizing the original Wuhan strain, certain variants are able to escape. At the same time, the study underlines the fact that the three key viral mutations, which SARS-CoV-2 seems inherently prone to develop, do not alter other vulnerable sites on the virus outside the receptor binding site.
This suggests that future vaccines and antibody-based treatments could provide broader protection against SARS-CoV-2 and its variants by eliciting or utilizing antibodies against parts of the virus besides the receptor binding site. The researchers note that broad protection against variants may be necessary if, as seems likely, the virus becomes endemic in the human population.