In vivo reprogramming therapy company Mogrify and Astellas Pharma, a developer of regenerative medicines, today announced a collaboration to combine their approaches to therapy development to discover and develop in vivo regenerative medicine treatment approaches to address sensorineural hearing loss (SNHL).
SNHL is caused by damage to the structures to the inner ear or the auditory nerve and is the cause of more than 90% of hearing loss in adults. Common causes of SNHL include exposure to loud noises, genetic factors, or the natural aging process. An estimated 1.57 billion people globally suffer from hearing loss, and a 2016 study in the American Journal of Public Health suggests more than 10% of people in the U.S. have severe to profound sensorineural hearing loss in at least one ear.
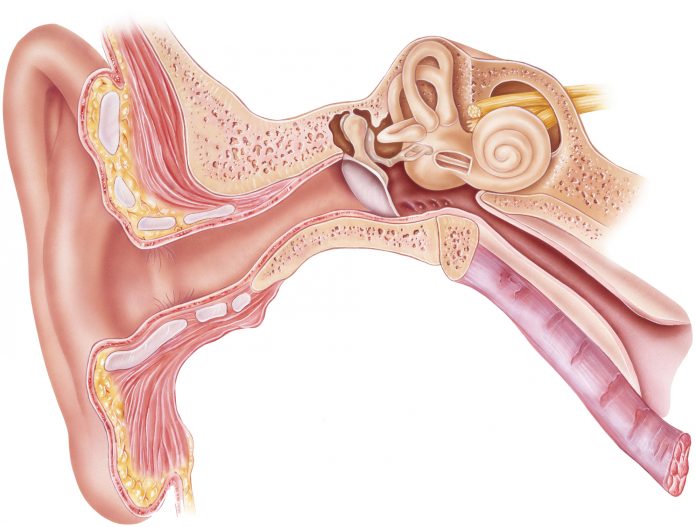
The collaboration will focus on the regeneration of cochlear hair cells, the tiny hairs found in the inner ear that translate vibrations to the auditory nerve to enable hearing. Cochlear hair cells can be damaged by repeated exposure to sounds greater than 85 decibels and also deteriorate as a process of normal aging.
Mogrify will apply its direct cellular reprogramming platform to identify combinations of transcription factors involved in cell differentiation to produce new cochlear hair cells. The company takes a big data approach to unravel direct cellular reprogramming and the maintenance of cell identity. It’s approach, refined over the course of 12 years, uses next-generation sequencing data, gene regulatory data, and epigenetic network data to prediction the transcription factors and growth factors required to produce any target human cell type from any source human cell type.
“Mogrify’s human regulatory network-centric approach is well placed to identify superior factor combinations, therefore increasing the efficiency of direct conversion toward the target cell type in the ear,” said Dr. Louise Modis, Mogrify’s CSO. “Combined with Astellas’ capabilities for gene therapy and research of sensorineural, this provides a clear path for the development of a novel in vivo reprogramming therapy for sensorineural hearing loss.”
Astellas will participate in the collaboration with Mogrify through its Astellas Gene Therapies Center of Excellence division. The company will fund the ongoing research costs and will also leverage its expertise in adeno-associated virus (AAV) based genetic medicine and translational capabilities to complete experiments in pre-clinical models, while Mogrify will screen and validate potential therapeutics.
Astellas Gene Therapies has ongoing research programs targeting X-linked myotubular myopathy, Pompe disease, and Myotonic dystrophy type 1. It is currently taking three different approaches to develop therapies for these three diseases: gene replacement, exon skipping gene therapy, and vectorized RNA knockdown. The program with Mogrify addresses a larger market than its current efforts that are focused on rare diseases.
“In this collaboration, we will look to combine the unique delivery attributes of AAV-based gene therapy, with our deep translational capabilities in otology developed through our ‘Targeted Therapeutics for Auditory Regeneration,’ and ‘Direct Reprogramming (Transdifferentiation)’ initiatives,” said Dr. Mathew Pletcher, senior VP, division head of Gene Therapy Research & Technical Operations at Astellas. “Through this collaboration, we will seek to address a significant unmet need in sensorineural hearing loss.”