Sponsored content brought to you by
Exploratory work needs to be cost effective and data must be generated quickly to support rational target discovery and biomarker identification. Biomarkers are crucial to the development of precision medicine treatments and companion diagnostics. But, in practice, it can be difficult distinguishing between potential therapeutic targets and phenotypic measures of disease. This distinction is particularly important in immuno-oncology applications where it is critical to understand whether the cells containing a biomarker of interest are tumor cells versus cells within the tumor microenvironment.
The Integral and Evolving Role of IHC
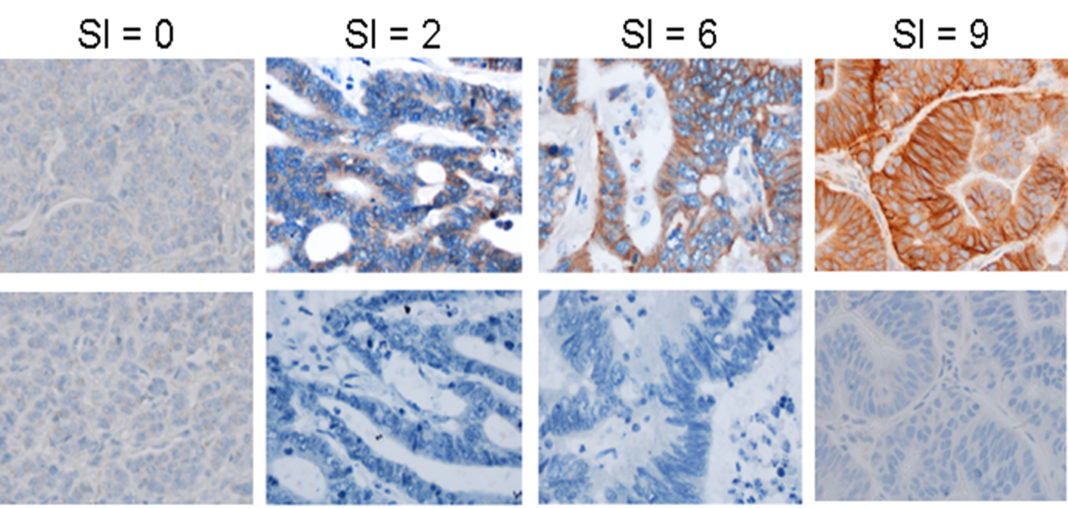
Immunohistochemistry (IHC) is a technology that combines immunological, biochemical, and anatomical techniques to image discrete components in tissues using labeled antibodies. With IHC, it is possible to visualize and document the distribution and localization of target components both within cells and within their histological context. Increasingly, IHC-based tissue biomarkers are being used in R&D for diagnosing disease, studying mechanism of action, selecting patients, or measuring/monitoring drug response.

To gain insight into how researchers are using IHC to drive cost-effective R&D, we spoke with Cullen Taylor, MD, Medical Director at Precision for Medicine, Biospecimen Solutions about the IHC lab he runs as a part of the CRO.
“IHC is an affordable and effective format that can be used to build the data necessary for running a successful early research program,” said Taylor. “Recognizing the importance of biomarkers for advancing exploration, we have built an integrated histology program that combines an ISO 9001-certified Biorepository with IHC capabilities and advanced downstream technologies to generate relevant, meaningful data.”
IHC is useful for informing the selection of appropriate biospecimens, which are a cornerstone of preclinical research. However, purchasing biospecimens with unknown biomarker results for biomarker screening can be cost prohibitive, preventing research objectives from being met. Precision for Medicine solves this by offering IHC services to prescreen cohorts of FFPE candidates for specific biomarkers to identify relevant biospecimens and data sets. For less than 20% of the cost of a biospecimen cohort, IHC testing of custom tissue microarrays (TMAs) can help researchers home in on the specimens that will help them answer their specific scientific questions.
TMAs allows screening at scale using formalin-fixed, paraffin-embedded (FFPE) donor blocks. Using the 3DHISTECH TMA Master, hundreds of tissues can be screened in a controlled environment simultaneously by a single technician, permitting exploration of the performance of biomarkers across broad sample subsets. At Precision for Medicine, TMAs are produced on demand with each core individually selected by board-certified pathologists to meet a researcher’s unique objectives.
“We have the tissue samples, TMA capability and IHC platforms all under one roof, streamlining the process,” explained Taylor. “We can onboard and optimize an assay, fully validate it, and then use it to cost-effectively screen hundreds of samples for the biomarker of interest. Not only are the services efficiently integrated but it is also a great entry point to learn more about your biomarker early on in development activity. Additionally, whole slide digitization using our Philips Ultra Fast Scanner enables us to share results efficiently with our clients around the globe”
Turnaround time is 48 to 72 hours for data generation and an additional 48-72 hours for interpretation and annotation by the company’s interdisciplinary pathology group.
Custom Biomarker Development
As part of their ongoing inventory gathering, Precision for Medicine screens the samples in their biorepository for relevant biomarkers, but most work is undertaken for specific needs. For instance, a researcher might have a new monoclonal antibody and a specific epitope they want to look at in different disease indications. The company’s laboratory houses leading IHC platforms from three of the major manufacturers (Roche, Leica, and Dako), enabling evaluation of an antibody’s performance across platforms for method comparison in vitro diagnostic (IVD) studies.
“There is increasing demand for IHC-based tissue biomarkers,” added Taylor. “We have the cross-functional expertise to support research from exploration all the way through to clinical trials and 510(k) submissions as biomarker assay requirements change with each phase of drug development.”
The team of scientists at Precision can handle all aspects of IHC biomarker analysis, including custom assay development, qualification and validation; sample collection logistics; global deployment of custom kitting; sample receipt and tissue processing; and companion diagnostic development.
The company also offers a spectrum of complementary or additive technologies that enable further characterization of samples. As the number of validated biomarkers grows, the ability to generate significant data from a limited amount of tissue is key. Multiplex immunofluorescence (IF) allows for fully quantitative simultaneous detection of up to eight distinct target proteins in the same FFPE tissue with single-cell resolution, while preserving sample material. This technology not only provides information on biomarker expression levels, but also increases the number of biomarkers that can be visualized at the same time.
Multiplex IF also has the capacity to provide information on the spatial distribution and activation state of different immune cell populations within a sample, which is useful for exploring the architectural context of the tumor microenvironment in oncology research.
“We understand the need to maximize the data generated from small amounts of tissue,” said Taylor. “Our histology program is built for purpose and provides meaningful annotated data sets quickly and professionally to move research forward.”