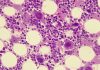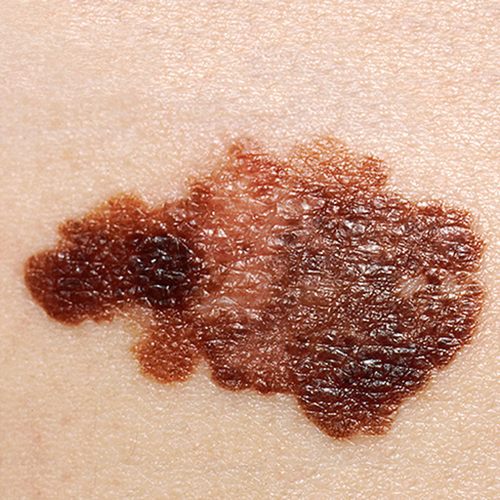
A pioneering test that reliably predicts the spread or return of melanoma has been developed by a team of Newcastle University scientists and clinicians. The research describing the test’s biological underpinnings was published Nov. 13 in the British Journal of Dermatology.
In this study, the researchers detail how early-stage melanomas at risk of spreading secrete a growth factor, TGFβ2, that causes downregulation of the proteins AMBRA1 and Loricrin—both of which are found in the skin overlaying the tumor. TGFβ2 also causes the loss of claudin-1, leading to loss of skin integrity and ulceration.
The study was led by Professor Penny Lovat at Newcastle University, U.K., in association with spin out company AMLo Bioscience.
When applied to the standard biopsy of a primary melanoma, the AMBLor test (named for the two proteins that are downregulated) identifies patients who are at low risk of the disease reoccurring or spreading. The test helps better determine a patient’s risk of disease progression for anyone diagnosed with a non-ulcerated early-stage melanoma—accounting for around 75% of all new diagnoses.
“TGFβ2 could also be a target to stop metastatic spread,” Lovat told Inside Precision Medicine. Lovat is Professor of Cellular Dermatology and Oncology at Newcastle University and Chief Scientific Officer at AMLo Biosciences.
Melanoma is increasing worldwide and every year more than 16,000 people in the UK and 96,000 people in the US are diagnosed with the cancer.
Lovat added that, “Around 91% of all melanomas diagnosed are AJCC [American Joint Committee on Cancer] stage I or II with fewer than 20% progressing. However, at present AJCC staging is unable to identify genuinely low risk subsets—meaning all cases are followed up as if high risk which places a huge burden of health care provision and resources as well as patient anxiety. Identifying those at genuine low risk of progression will ease patient anxiety, reduce waiting lists and save on healthcare resource.”
In a press release, Lovat explained that “Like mortar and bricks holding together a wall, AMBRA1, Loricrin and Claudin 1 are all proteins key to maintaining the integrity of the upper layer of the skin. When these proteins are lost gaps develop – like the mortar crumbling away in the wall. This allows the tumor to spread and ultimately ulcerate which we know is a process associated with higher risk tumors. Our new understanding of this biological mechanism underpins the test we have available.”
Lovat added, “This test will aid clinicians to identify genuinely low risk patients diagnosed with an early-stage melanoma and to reduce the number of follow up appointments for those identified as low risk, saving NHS time and money.”
Currently, primary tumors are removed by surgery and pathologists study the biopsy under the microscope to determine the stage the skin cancer is at and the risk of it spreading (metastasis). Even if defined as low risk, the patient is followed up in clinic for as long as five years. It is these low-risk patients that the test identifies.
The AMBRA1 and loricrin test is approved by the UK Accreditation body (UKAS) and is already available through a private referral service from AMLo Biosciences. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis.