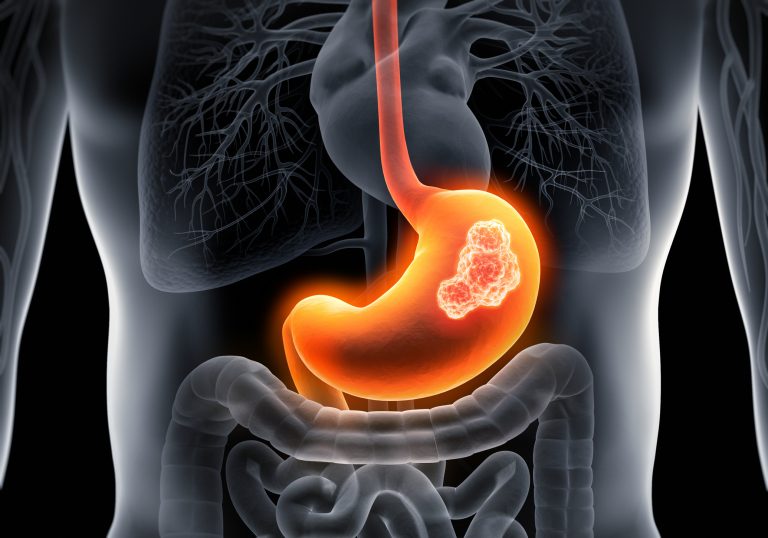
The FDA has approved Bristol-Myers Squibb’s monoclonal antibody Opdivo (nivolumab) for first-line treatment of gastric cancer in combination with fluoropyrimidine- and platinum-containing chemotherapy.
Cancer cells release proteins that can protect themselves from immune T cells that are seeking to destroy them. Nivolumab is a checkpoint inhibitor drug and acts by blocking these protective proteins (PD-1) so the patient’s T cells are able to destroy the cancer cells.
Nivolumab has previously been approved to treat a number of different cancers including as a first-line treatment for some types of melanoma, but this is the first time any immunotherapy has been approved as an initial treatment for gastric cancer.
“Today’s approval is the first treatment in more than a decade to show a survival benefit for patients with advanced or metastatic gastric cancer who are being treated for the first time,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“The FDA is committed to bringing new safe and effective treatment options like Opdivo to patients with advanced cancer.”
Gastric cancer is the fifth most common type of cancer around the world, with approximately 28,000 new cases each year in the U.S. It is the third leading cause of death from cancer around the world with a 5-year survival rate for advanced or metastatic gastric cancer of only 5%.
The new approval is based on a randomized, open label trial of 1,581 patients with previously untreated, advanced or metastatic gastric or esophageal cancer. In the trial 789 patients received nivolumab in combination with chemotherapy and 792 received chemotherapy alone.
The addition of nivolumab to their treatment regimen, increased survival time for patients. At 1 year, 55% of patients in the nivolumab arm were still alive versus 48% on chemotherapy alone. Overall survival on nivolumab was improved by 20% and 29% in patients with a positive PD-L1 score.
Risk of disease progression or death was also reduced by 32% in the nivolumab group compared with chemotherapy alone.
Nivolumab can cause a number of different side effects including peripheral neuropathy, nausea, fatigue and several others, including more severe inflammatory side effects linked to the immune system such as inflammation of the lungs or the colon.
This approval was granted after Bristol Myers Squibb received Priority Review and Orphan Drug designations from the FDA for nivolumab for this indication. The review was also part of the FDA’s Project Orbis initiative, which allows the health authorities in Canada, Australia, Switzerland and Brazil to review drugs at the same time as the FDA.
“We are focused on bringing transformative medicines to patients in need, and historically, there has been little progress for patients diagnosed with these metastatic gastroesophageal adenocarcinomas,” said Adam Lenkowsky, general manager and head, U.S., Oncology, Immunology, Cardiovascular, Bristol Myers Squibb.
“Opdivo is the first and only immunotherapy combined with chemotherapy to deliver superior overall survival versus chemotherapy alone in first-line metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.Today’s approval may offer these patients hope for the chance at a longer life.”