Scientists have identified four promising antiviral drug candidates, including the FDA approved hepatitis C drug boceprevir, that show strong potential for treating COVID-19.
The other three drug candidates that showed anti-viral potential included a, as yet unapproved, drug being investigated to treat a strain of coronavirus in cats and two inhibitors of the protein calpain, which has been linked to increased risk of blood clots at high altitude. Calpain inhibitors have been trialled for treatment of cancer, neurodegenerative diseases and other conditions in the past, but are not currently approved for treatment of any condition.
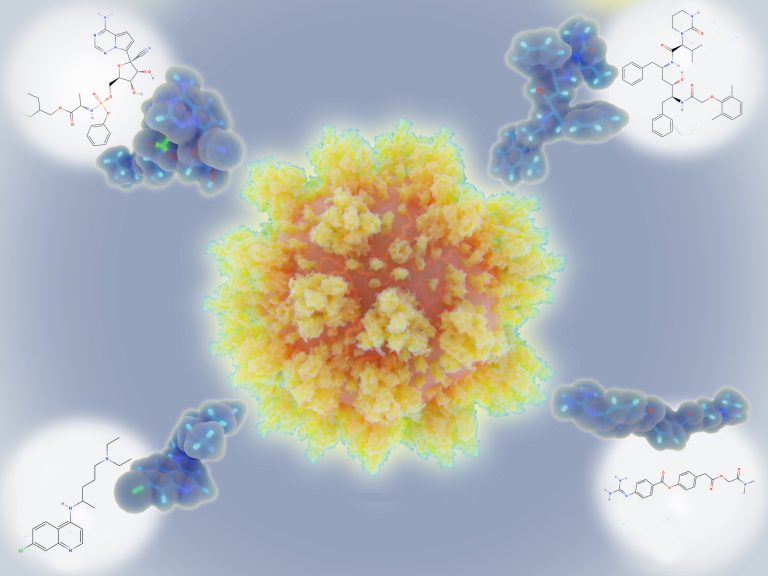
The researchers, based at University of South Florida Health and the University of Arizona College of Pharmacy, worked together to screen 55 possible antiviral compounds to assess their ability to treat SARS-CoV-2.
The compounds the team selected were chosen for their ability to inhibit a viral enzyme called the main protease in cells infected with the virus in the laboratory. This enzyme plays a key role in viral replication so blocking its activity should stop the virus from replicating and infecting other cells. Inhibition of this enzyme has been shown to reduce viral replication of both SARS and MERS, which are also coronaviruses.
Adverse effects can be a significant problem for some antiviral drugs, so this is a concern for developers. One advantage of targeting the main protease of SARS-CoV-2 is that there is not an equivalent enzyme in humans, meaning possible side effects should be minimized.
As reported in the journal Cell Research, the researchers found that these four compounds were all able to significantly inhibit viral replication in the infected cells, although the drug candidate that had already been investigated for its ability to treat coronavirus in cats had the most potent antiviral activity. According to the researchers, all four of the candidate antivirals had more significant activity against the protease than similar candidates that have already been assessed for their ability to inhibit replication of SARS-CoV-2.
The current COVID-19 pandemic has had wide reaching impact worldwide. Although the race to produce the first vaccine is going at an unprecedented speed, it will likely be many months before an effective vaccine is approved. There is a real need for better treatments, particularly for those badly affected by the virus, and developing an effective antiviral would make a big difference to many patients.
A big advantage of boceprevir, one of the four candidate drugs, is that it is already approved by the FDA to treat hepatitis. This means that while it would still have to undergo testing before it could be given to COVID-19 patients, it could potentially reach the clinic quicker, as its safety and tolerability is already known.
“With a rapidly emerging infectious disease like COVID-19, we don’t have time to develop new antiviral drugs from scratch,” said Yu Chen, PhD, University of South Florida Health associate professor of molecular medicine and a senior author of the study article.
“A lot of good drug candidates are already out there as a starting point. But, with new information from studies like ours and current technology, we can help design even better (repurposed) drugs much faster.”













