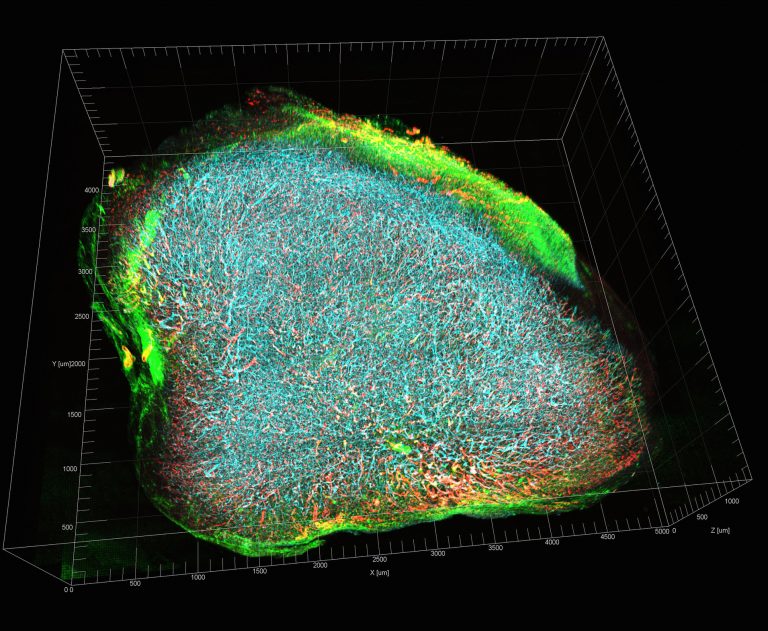
A novel approach called nano-radiomics that utilizes complex analyses of imaging data to assess changes in the tumor microenvironment (TME) that cannot be detected with conventional imaging methods was recently developed by researchers led by scientists at the Baylor College of Medicine and Texas Children’s Hospital.
Their study, “Detection of response to tumor microenvironment–targeted cellular immunotherapy using nano-radiomics”, published in Science Advances, provides the promise of a new noninvasive means to enhance current imaging methods in measuring and monitoring the effectiveness of cellular immunotherapies designed to specifically target the TME, according to the team.
“Immunotherapies, including cell-based therapies, targeting the tumor microenvironment (TME) result in variable and delayed responses. Thus, it has been difficult to gauge the efficacy of TME-directed therapies early after administration. We investigated a nano-radiomics approach (quantitative analysis of nanoparticle contrast–enhanced three-dimensional images) for detection of tumor response to cellular immunotherapy directed against myeloid-derived suppressor cells (MDSCs), a key component of TME,” write the investigators.”
“Animals bearing human MDSC-containing solid tumor xenografts received treatment with MDSC-targeting human natural killer (NK) cells and underwent nanoparticle contrast–enhanced computed tomography (CT) imaging. Whereas conventional CT-derived tumor metrics were unable to differentiate NK cell immunotherapy tumors from untreated tumors, nano-radiomics revealed texture-based features capable of differentiating treatment groups.”
“Our study shows that TME-directed cellular immunotherapy causes subtle changes not effectively gauged by conventional imaging metrics but revealed by nano-radiomics. Our work provides a method for noninvasive assessment of TME-directed immunotherapy potentially applicable to numerous solid tumors.”
“Understanding the response of the tumor microenvironment to anti-cancer therapy is becoming increasingly important,” said co-corresponding author Robin Parihar, MD, PhD, assistant professor of pediatric hematology-oncology at Baylor and Texas Children’s and a member of Baylor’s Center for Cell and Gene Therapy, “particularly when the tumor microenvironment is inhibiting the anti-tumor effectiveness of cellular immunotherapies that are engineered to attack the cancer.”
Currently, imaging technologies such as computed tomography (CT) or magnetic resonance imaging (MRI) generate three-dimensional images that provide information about the overall tumor response to therapy, for instance, whether it is growing or shrinking, but provide very little, if any, information about the TME, note the scientists.
Parihar approached Ketan Ghaghada, PhD, assistant professor of radiology at Baylor and a member of the Translational Imaging Group (TIGr) at Texas Children’s, and their laboratories began a collaboration to develop a noninvasive method to assess the effect of a cellular immunotherapy treatment specifically directed at the TME.
Parihar, Ghaghada, and their colleagues developed nano-radiomics, a technique that combines imaging technology using a nanoparticle contrast agent, with radiomics for computational mining of 3-D imaging data.
“Radiomics is an emerging area in the field of radiology wherein images are analyzed to extract information that may reveal patterns or textures in the tumor that are not visible to the naked eye. To enhance the quality of the images, we used a nanoparticle contrast agent developed in our lab that has a different pattern of distribution within the tumor than traditional contrast agents, one that is indicative of changes in the TME,” said Ghaghada, co-corresponding author of this work and a member of Baylor’s Dan L Duncan Comprehensive Cancer Center.
Using mouse models of cancer, the researchers treated one group of animals with a cellular immunotherapy that effectively eliminated the TME and another group with a placebo. Both groups received the nanoparticle contrast agent followed by a CT scan. Radiomics analysis of the imaging data of both groups showed that nano-radiomics revealed texture-based features that distinguished the two groups while traditional CT scans did not, suggesting that this new approach has the potential to enhance the ability of clinicians to noninvasively assess the effect of treatments directed at the TME, ultimately enhancing the impact of cancer treatment and management.