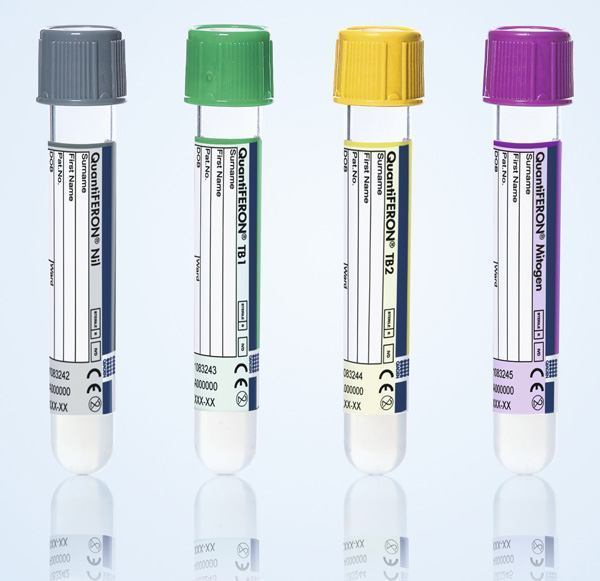
Qiagen and DiaSorin said they have begun the U.S. commercial launch of an automated workflow for their QuantiFERON-TB Plus (QFT-Plus) diagnostic for use on DiaSorin’s LIAISON platforms, following FDA approval of the test for detecting latent tuberculosis (TB).
The LIAISON QuantiFERON-TB Plus Test is designed to offer streamlined laboratory automation for latent TB screening, supporting conversion from century-old tuberculin skin tests to fourth-generation blood-based QuantiFERON technology.
Qiagen and DiaSorin said the automated workflow on LIAISON platforms is intended to offer QuantiFERON customers a powerful, highly flexible automation option for all throughput ranges. Embedding QuantiFERON assays within the assay menu of DiaSorin’s LIAISON analyzers is also designed to give current LIAISON customers a new assay option with significant growth potential.
The new workflow pairs QIAGEN’s standard QuantiFERON-TB Gold Plus Blood Collection Tubes (QFT-Plus BCT, containing the core QuantiFERON technology) with DiaSorin’s newly launched LIAISON QuantiFERON-TB Plus detection assay.
More than 8,000 LIAISON systems have been placed worldwide, primarily in hospital laboratories, according to the companies.
“The validation of the QuantiFERON technology with the LIAISON platforms further reinforced the clinical profile of QuantiFERON-TB Gold Plus,” Thierry Bernard, Interim CEO of Qiagen and Senior Vice President, Head of the Molecular Diagnostics Business Area, said in a statement. “QFT-Plus users are gaining access to the LIAISON’s powerful, highly flexible automation for all throughput segments, as well as to LIAISON’s broad menu of more than 100 tests.”
Qiagen and DiaSorin launched their collaboration in 2017, with the goal of developing new tests for the LIAISON family of analyzers based on Qiagen assay technologies. The companies said they plan to adapt to the LIAISON platforms additional tests based on QuantiFERON technology, which is designed to provide an efficient method of detecting asymptomatic infections and other risks that may not be detected with standard diagnostic technologies.
The U.S. launch follows the introduction of the LIAISON workflow for QFT-Plus in September 2018, with plans to roll out the new automated workflow to China in 2020.
In addition to its partnership with DiaSorin, Qiagen is collaborating with two developers of liquid handling solutions to provide options for automated pre-analytical processing for customers who implement the single-tube collection process for QFT-Plus. The collaborations are meant to enable customers to automate the manual step of aliquoting samples from a single collection device into QuantiFERON-TB Gold Plus tubes for analysis, using either the Hamilton Robotics Microlab STAR automated liquid handling workstation or the Tecan Fluent Laboratory Automation workstation.
In addition to the U.S., QuantiFERON-TB Gold Plus is registered in more than 75 countries in Europe, Asia, Africa, and Latin America, as well as North America. QFT-Plus also has the future potential to deliver increased clinical utility by adding measurement of CD8+ T-cell immune response to detection of CD4+ response, Qiagen and DiaSorin said.
According to Qiagen and DiaSorin, the LIAISON workflow for QFT-Plus delivers “significant” gains in turnaround time and efficiency when combined with front-end automation options for liquid handling.
“We believe this solution for laboratories will furtherly strengthen our positioning as a specialty player,” DiaSorin Group CEO Carlo Rosa added.