With more than a dozen immune checkpoint inhibitor drug candidates in development that target the LAG-3 protein, there is still scant information on the its structure and function. Now, researchers at Stanford University and New York University (NYU) have discovered new insights into LAG-3. Their research, published in Proceedings of the National Academy of Sciences, contains key details of the molecule’s structure, as well as information about how the LAG-3 protein functions.
“Given the amount of time and resources being put into developing therapeutics that target LAG-3, it is astounding that we don’t yet have a full understanding of how this protein functions,” said Jennifer Cochran, the Addie and Al Macovski Professor in the School of Engineering and professor of bioengineering, and co-senior author on the study.
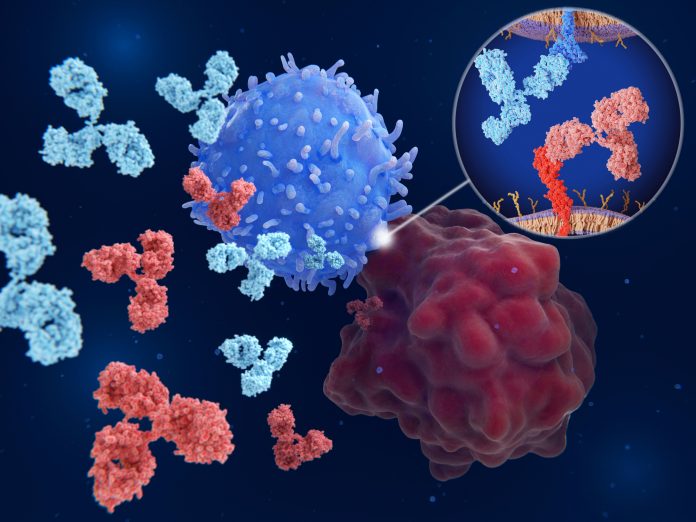
Proteins such as LAG-3 exist to stop our immune system from attacking things they shouldn’t. In theory, our immune system should naturally recognize tumor cells as foreign. But a checkpoint protein shield can give cancer cover.
Current immunotherapies aren’t chemical drugs, they’re lab-manufactured antibodies that attach to certain parts of these checkpoints, and essentially turn them off. Once the checkpoint is turned off, our immune system can recognize and target the cancer again.
There are already approved antibody treatments that target two checkpoint proteins: CTLA-4 and PD-1. Both turn off our immune systems but in different ways. Because CTLA-4 and PD-1 were the first two checkpoint proteins found, they are quite well studied, and different approaches to inhibiting them for cancer therapy earned scientists the 2018 Nobel Prize in physiology or medicine.
LAG-3 seems to work in an entirely different way in comparison to CTLA-4 and PD-1. Scientists hope that those differences might make it a better or complementary target to treat certain types of cancer, said Jack Silberstein, a Stanford immunology PhD student who co-led the work.
Because of that, Silberstein said, “there was all this excitement in the field. Groups rushed to make antibodies against LAG-3, without knowing entirely how LAG-3 or those antibodies functioned.”
Additional work by the team uncovered, for the first time, that an antibody that has been used for close to 20 years to demonstrate therapeutic efficacy in animal tumor models blocks the activity of LAG-3 by binding to the interface between two LAG-3 molecules, disrupting LAG-3 from forming its functional dimer.