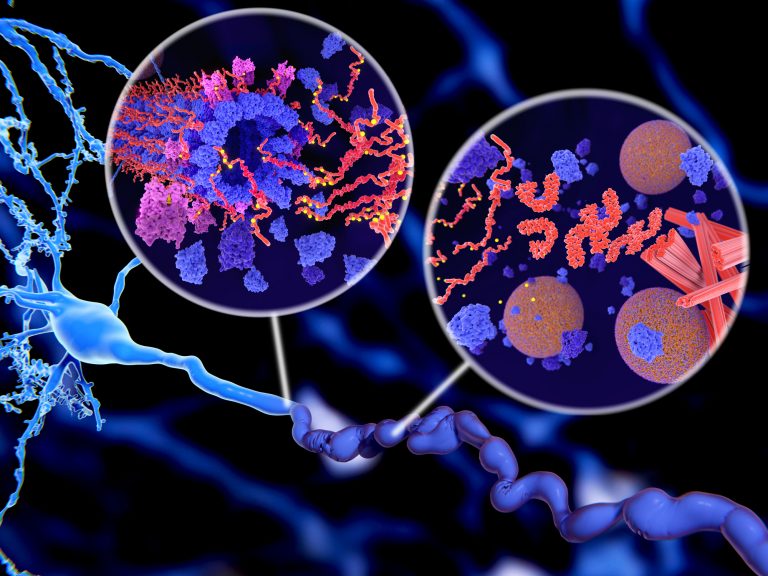
A molecular pathway in Alzheimer’s disease (AD) that could lead to the development of new therapeutic approaches to target the neurodegenerative condition early in its progression was discovered recently by researchers at Case Western Reserve University School of Medicine. The team’s studies, in cells from human AD patients and in mouse models of the disease, indicated that the pathway, known as Drp1-HK1-NLRP3, plays a key role in disrupting the normal function of brain cells that produce the protective white matter myelin sheath that wraps around neurons.
“This is a missing part of the puzzle,” said research lead Xin Qi, PhD, a professor in the department of physiology and biophysics at the School of Medicine. “We’ve discovered a pathway that is accessible to detection and potential treatment, prior to much of the disease’s damage and well before clinical symptoms appear.” Reporting on their findings in Science Advances (“Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease,”) Qi and colleagues concluded, “Overall, our findings provide previously unidentified insights into the mechanism of white matter degeneration in AD and thus identify a new area for potential therapeutic intervention … The Drp1-HK1-NLRP3 signaling axis may be a key mechanism and therapeutic target for white matter degeneration in AD.”
First identified more than 100 years ago, AD is an age-related neurodegenerative disorder that is characterized by deposits of β-amyloid (Aβ) and neurofibrillary tangles (NFTs) in the brain, the authors wrote. “Before plaques and tangles become apparent in AD patients, white matter loss and demyelination are evident, often manifesting early in the course of the disease and predicting disease status.” While the cause of AD is not known, the greatest risk factors for developing the disease are age, genetics, and a previous traumatic brain injury.
The dysfunction and eventual death of myelin-producing oligodendrocytes are well-established early events in AD that lead to cognitive deficits. “Such brain injury is attributable to the damage of oligodendrocytes (OLs), myelin-forming glial cells in the central nervous system,” the team continued. However, the underlying mechanism behind myelin degradation and white matter loss resulting from OL death isn’t known. “There is a growing body of evidence in the field that AD develops much earlier than previously thought, most likely decades before our current ability to clinically diagnose the condition,” said study co-author Andrew A. Pieper, Ph.D., the Morley-Mather chair in neuropsychiatry in the School of Medicine and director of the Harrington Discovery Institute Neurotherapeutics Center at University Hospitals.
The researchers’ reported studies have now found that the Drp1-HK1-NLRP3 pathway plays a key role in disrupting the normal function of brain cells that produce myelin, and illuminate how OLs start to go awry, due to overexpression of the protein (Drp1) within the recently discovered pathway. The findings suggest that hyperactivation of Drp1 triggers inflammation and injury to OLs, culminating in a reduction of myelin, and a slowing communication in the brain, leading to degeneration of white matter and significant cognitive impairment.
The investigators validated their discovery of the pathway using mouse models of AD and post-mortem brain samples of AD patients. In their published study, the researchers found that eliminating Drp1 expression in mouse models corrected the energy-related defect in OLs associated with hyperexpression of the protein. “This approach also reduced the activation of inflammation OLs, lessened tissue damage at those brain sites and improved cognitive performance,” they wrote. “ … our findings have revealed a Drp1-HK1-NLRP3 signaling axis in mature OLs that elicits metabolic stress in OLs, thereby triggering inflammation and OL injury that culminates in demyelination, white matter degeneration, and cognitive impairment in models of AD.
“Detecting the disease—and potentially treating it—at earlier stages will be critical to our battle against its devastating effects,” said Pieper, who is also a psychiatrist at the Louis Stokes Cleveland VA Medical Center Geriatrics Research Education and Clinical Center (GRECC). “The new pathway uncovered by Qi’s laboratory could be targeted for therapy before the disease has progressed to the point of causing cognitive problems.”
A near total degeneration of OLs occurs before common symptoms of AD become apparent in most patients, so the researchers hope to target and manipulate the pathway with therapeutics that regulate the expression of Drp1, thereby slowing or reducing damage to myelin-producing OLs. Qi’s lab has already patented a small molecule peptide inhibitor that regulates the expression of Drp1, effectively putting the brakes on degeneration of brain cells.
“Our results show promise that targeting the Drp1-HK1-NLRP3 pathway and reducing the expression of the Drp1 protein could help reduce the downstream cascade of abnormal brain functions associated with the progression of AD,” said Qi, whose lab has studied Drp1 for a decade, mostly in Parkinson’s disease and Huntington’s disease. “If therapies targeting this pathway can slow, stop, or even reverse early-stage AD progression, then possibly there can be a reduction or delay to later stage damage and impairments.”
Most AD diagnoses are in patients 65 or older, so identifying the disease in younger patients can be difficult. Many patients experience a significant loss in their brain’s white matter—which is central to cognition, emotion, and consciousness—before receiving a diagnosis. “Identifying how AD unfolds in its earliest processes will help scientists better understand how to focus research into potential solutions for patients,” continued Pieper. “The Qi lab’s findings may help in targeting AD earlier, potentially leading to better management of its symptoms and progression … There have still been only a very small number of approved medications for AD since its discovery in 1907. While these medicines augment neurotransmission to provide temporary symptomatic benefit, they do nothing to slow disease progression. Identification of earlier approaches to treating AD—such as this research—is critical for society as the magnitude of AD is growing explosively with our aging population.”