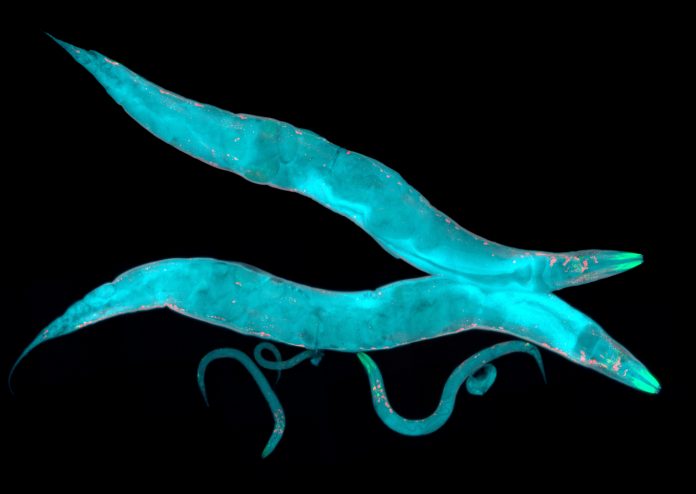
A new study at Canada’s CHUM Research Centre (CRCHUM) has found that the probiotic bacterium Lacticaseibacillus rhamnosus HA-114 prevents neurodegeneration in C. elegans, a model used for the study of amyotrophic lateral sclerosis (ALS).
The findings, from the lab of Alex Parker a professor of neuroscience at the Université de Montréal and published in the journal Communications Biology, suggests that the disruption of lipid metabolism contributes to cerebral degeneration. Further, it shows that the non-commercial probiotic HA-114 was unique in its ability to provide neuroprotection compared to other strains of bacteria tested in the study.
“Recent research has shown that the disruption of the gut microbiota is likely involved in the onset and progression of many incurable neurodegenerative diseases, including ALS,” said lead author Parker. “When we add (HA-114) to the diet of our animal model, we notice that it suppresses the progression of motor neuron degeneration. The particularity of HA-114 resides in its fatty acid content.”
First author on the study was Audrey Labarre, and ALS researcher who works in the Parker lab. Labarre has been working with C. elegans for research on motor neuron degeneration. For this study, she genetically modified the worms with ALS-associated genes, then tested 13 bacterial strains and three strain combinations to study their effects. HA-114 had a significantly larger effect than any of the bacterial strains, or strain combinations in reducing motor disorders in ALS, Huntington’s disease, and other neurodegenerative disease models.
“We believe that fatty acids supplied by HA-114 enter the mitochondria through an independent, non-traditional pathway,” Parker explained. “In doing so, they restore balance to impaired energy metabolism in ALS and lead to a decrease in neurodegeneration.”
Using data from the genetic study, genomic profiling, behavioral analysis and microscopy images of the models, the researchers also identified two genes, acdh-1 and acs-20, which play a key role in this neuroprotective mechanism.
Existing in equivalent forms in humans, both genes are involved in lipid metabolism and beta-oxidation, a process through which fatty acids are broken down into energy in the mitochondria, the true cellular power plants.
With the encouraging findings in hand, the research team has now begun to test HA-144 in mouse models with an eye toward eventually moving to in-human clinical studies to determine if Ha-114 could be a viable therapeutic complement to existing ALS therapies.