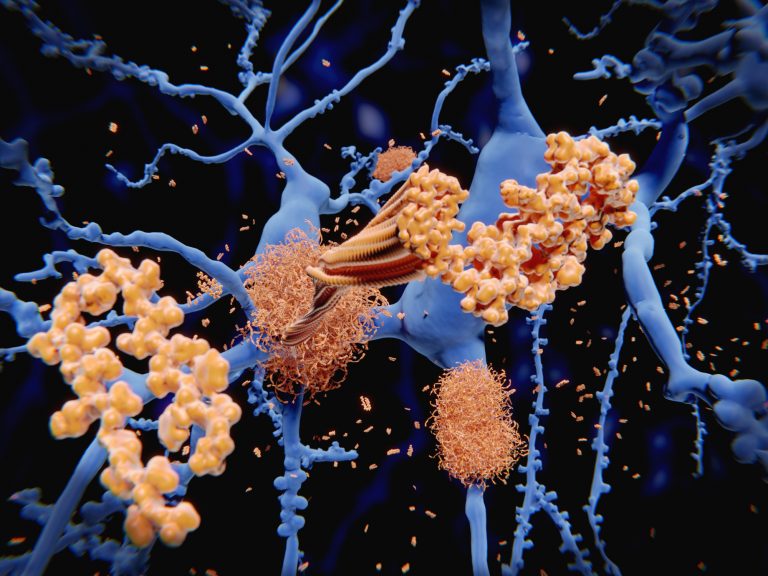
Although there have been many studies done, there remain many questions on the exact Amyloid-beta (Aβ) form responsible for neurotoxicity in Alzheimer’s Disease. Now researchers have revealed for the first time the atomic structure of Aβ protein assemblies.
Their study, “Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage,” is published in Nature Communications and led by Natàlia Carulla, Institute For Research In Biomedicine (IRB) Barcelona Alumni, former group leader at the Institut Européen de Chimie et Biologie (IECB) in Bordeaux, and current project manager at Grup CIEF.
The discovery of this structure reveals a new mechanism of toxicity for these assemblies, revealing their capacity to disrupt the neuronal membrane, allowing water and ions to pass through it, and causing the death of these cells.
“Knowing the features that characterize these protein ensembles, such as the number of molecules that make them and the shape they adopt, is crucial to design effective therapeutic strategies that target the forms of Aβ ensembles responsible for the neurotoxicity in AD,” Carulla noted.
The team of researchers first studied the Aβ protein in vitro to ensure stable Aβ forms of uniform composition and shape. Then they studied their structure and mode of neurotoxicity, establishing a 3D arrangement of all the atoms making up the Aβ ensemble.
“Formation of Aβ oligomer pores in the membrane of neurons has been proposed to explain neurotoxicity in AD. Here, we present the three-dimensional structure of an Aβ oligomer formed in a membrane mimicking environment, namely an Aβ(1-42) tetramer, which comprises a six-stranded β-sheet core. The two faces of the β-sheet core are hydrophobic and surrounded by the membrane-mimicking environment while the edges are hydrophilic and solvent-exposed…These studies revealed a mechanism of membrane disruption in which water permeation occurred through lipid-stabilized pores mediated by the hydrophilic residues located on the core β-sheets edges of the oligomers,” the researchers wrote.
Their study suggested that some Aβ associations can perforate the neuron membranes and change their osmotic equilibrium, and eventually trigger their death. These findings give researchers a new understanding of Aβ protein assemblies in Alzheimer’s disease and neuronal death, and provides a new strategy and hope in treating AD.