“Geology is the study of pressure and time. That’s all it takes really… pressure… and time…,” bellows Morgan Freeman’s character, Red, when foreshadowing his friend Andy Dufresne’s harrowing escape from prison in the critically acclaimed movie Shawshank Redemption. Oddly enough, this same logic can be applied to antibiotic resistance, although bacterial change occurs exponentially more rapidly than the millennia it can take to create various geological wonders. For microbes, however, the biological pressures that scientists presume underlie the formation of resistance mutations doesn’t come in the form of sedimentation, stalactites, or shifts in plate tectonics, as it does in geology. Rather, the genetic burdens exerted by numerous human developed antibiotic, and drug therapies, as well as natural environmental conditions, are the most significant factors fueling resistance.
While antimicrobial drug resistance is growing at an alarming rate globally, the phenomenon is not new, nor is it an occurrence that only recently has come to pass. In 1940, only slightly a decade after penicillin’s discovery, the first strains of Staphylococcus resistant to the compound were detected. Five years later, in his Nobel lecture concerning his breakthrough discovery, Alexander Fleming prophetically warned those in attendance about the dangers of microbial resistance:
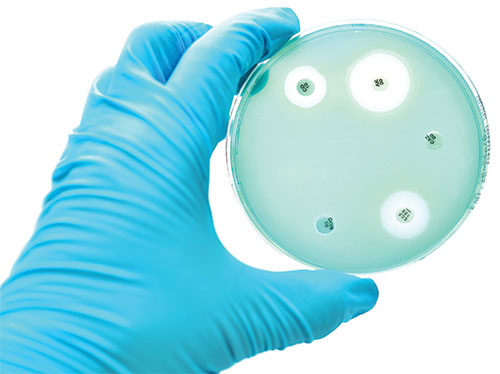
“It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body…The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant.”
While numerous factors are contributing to this sharp rise in resistance that the world is currently experiencing, chief among them is the overuse of antibiotic compounds. Given their extremely rapid and exponential replication rate (an E. coli
bacterium can double itself approximately every 20 minutes), and ability to survive an extensive number of genetic mutations, bacteria have evolved to be the ultimate survivor.
Certainly, long after humans have outlived their time on this planet, microbes will continue to chug along, barely even recognizing our species’ existence. Force the microscopic critters to choose between dying or rapidly adapting by placing them under the chemical squeeze of antibiotic compounds, and they will be all too happy to mutate and return even more virulent. Keep in mind that there are bacterial species (Deinococcus radiodurans) that can withstand a dose of ionizing radiation 3,000 times greater than the dose that would kill a human—due to their extreme ability to repair and adapt to DNA damage that would destroy most life.
Get in Front of It
So how does science keep on top of a such a seemingly formidable, if not tiny, adversary? Obviously, there are multiple approaches, but developing better therapeutics is paramount for most researchers. While compounds that are fast acting, exquisitely lethal, and unique to only pathogenic bacteria would be ideal, science isn’t quite capable of that form of therapy just yet. However, we are well entrenched in the genomic age, and investigators have advanced tools at their disposal which allow them to identify genotypic mutations that rapidly lead to resistance phenotypes.
“Genomic analysis offers an unprecedented window into the origins, functions, and dissemination of antibiotic resistance,” says Michael Gillings, Ph.D., professor in the department of biological sciences at Macquarie University, Sydney, Australia. “We now have the power to understand how antibiotic resistance arises in real time, how resistance genes move between bacterial species, and how bacterial species are spread around the globe. Submissions to globally accessible databases ensure that researchers everywhere can obtain information quickly.”
Gautam Dantas, Ph.D., associate professor in the department of molecular microbiology at Washington University School of Medicine thinks that the simplest impact OMICS is having on the study of resistance is “the ability for us to transcend the culturing barrier. So much of microbiology and the functions encoded in microbiology, such as resistance, are based on ideas of domesticating microbes by bringing them into the lab and studying them deeply. While this method has been very useful, we’ve learned from soil and environmental researchers, as well as the human microbiome project and beyond, that most habitats have rich microbial communities that are not trivial to culture. So genomics sort of gets beyond that by going after the nucleic acids themselves and inferring what the functions might be—and the study of resistance has been expanded because of that approach.”
The influence of genomics on fields like microbial genetics is best viewed through the lens of advanced techniques such as next-generation sequencing (NGS), which has quickly evolved into an affordable method now employed in both research and clinical settings. For microbiologists tasked with monitoring mutations within microbial populations that could lead to resistance, being able to quickly and accurately assess bacterial genotypes is paramount in attemping to stay ahead of drug resistance outbreaks.
Click here to access the rest of this article.











