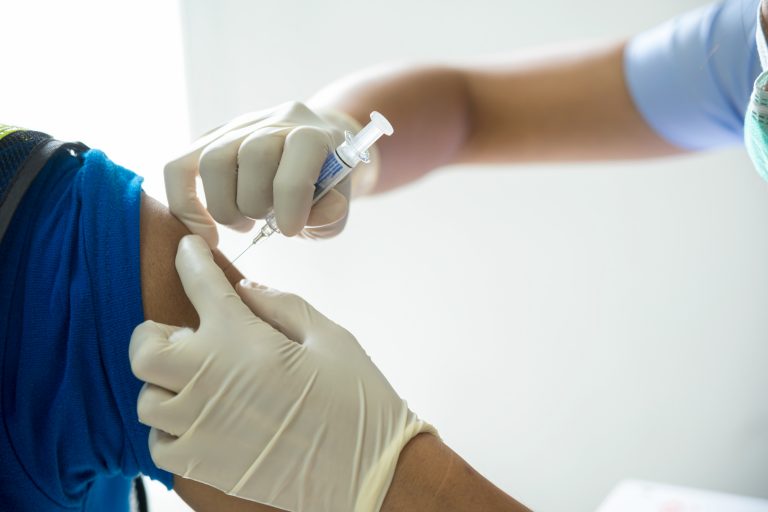
It’s a question that has hung over the fight against COVID-19 from the beginning: Will vaccination require booster shots to keep the pandemic at bay? The answer increasingly seems to be ‘yes,’ especially as more deadly and more easily spread strains of SARS-CoV-2 have emerged. The National Institutes of Health is now preparing for that eventuality by beginning a Phase I/2 clinical trial to test the safety and efficacy of different booster schedules.
“Although the vaccines currently authorized by the U.S. Food and Drug Administration offer strong protection against COVID-19, we need to prepare for the possibility of needing booster shots to counter waning immunity and to keep pace with an evolving virus,” said Anthony Fauci, M.D., Director of the National Institute of Allergy and Infectious Diseases. “The results of this trial are intended to inform public health policy decisions on the potential use of mixed vaccine schedules should booster doses be indicated.”
Forty-one percent of the U.S. population is fully vaccinated against COVID-19, translating to 296 million doses. By comparison, 175 million doses of flu vaccine were given in 2019.
Booster shots are not new to Americans. They are, however, more associated with childhood vaccines, such as those against measles, mumps and rubella — better known as the MMR vaccine. The Centers for Disease Control and Prevention recommends MMR vaccination in babies and boosters in children 4 to 6 years old, as well as for students attending college and adults who will be in settings or countries that pose high risk.
Current childhood vaccines contain live, replicating virus; often resulting in lifetime immunity. The current mRNA-based COVID-19 vaccines (Pfizer and Moderna) do not, which means their efficacy is likely to wane more quickly than other vaccines. Ongoing studies of vaccinated individuals are working to determine exactly if and when immunity falls to levels that no longer offer protection against SARS-CoV-2.
As part of the current NIH trial, adults who have been fully vaccinated with any of the three COVID-19 vaccines currently available in the U.S.—made by Pfizer, Moderna and Johnson & Johnson—will receive boosters made by Moderna. A total of 150 people will be enrolled and separated into six groups. Each vaccine group will enroll about 25 people ages 18 through 55 years and approximately 25 people age 56 years and older. Participants will receive a single booster dose of the Moderna vaccine as part of the trial 12 to 20 weeks following their initial vaccination regimen.
An additional cohort will be composed of adults who have not received an FDA authorized vaccine. These individuals will first receive two doses of the Moderna vaccine, followed by a booster 12 to 20 weeks later.
All participants will be followed for one year, meeting with investigators by phone and in-person. Periodic blood samples will be used to test immune responses against current circulating strains of the virus, as well as emerging variants. If a participants develops symptomatic COVID-19, genetic analysis will be performed to determine whether their infection was caused by a novel variant of the virus. Initial results are expected late this summer.
For more information about the trial, including a list of enrollment locations, please visit clinicaltrials.gov and search identifier NCT04889209.