Study results show that sugar molecules called glycans that coat the SARS-CoV-2 spike protein help it to bind to and infect human cells through the angiotensin-converting enzyme 2 (ACE2) receptor, something that could help drug and vaccine developers target the virus.
Reporting on their work in ACS Central Science, University of California, San Diego research leads, Lorenzo Casalino, PhD, and Zied Gaieb, PhD, and colleagues from the University of Texas at Austin, and Maynooth University in Dublin, Ireland, say their findings could lead to the identification of new drug targets, and point to “opportunities and challenges for small molecules and vaccine design.”
Their research is described in a paper titled, “Beyond Shielding: The Roles of Glycans in the SARS-Cov-2 Spike Protein.”
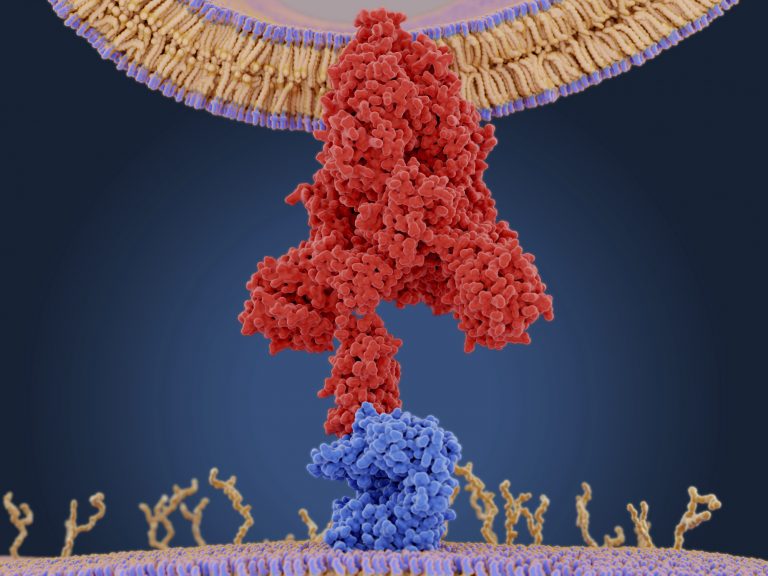
Coronaviruses, including SARS-CoV-2, are lipid-enveloped, positive-sense RNA viruses, the authors explained. “Together with the host-derived membrane, a set of structural proteins provides an organizational scaffold that wraps and contains the viral RNA.” Among these viral proteins, the most critical is the spike, or S protein, which is conserved to varying degrees across the Coronaviridae family of viruses and plays a key role in how the virus attaches to and fuses with the host cell.
Research focused on the development of vaccines and drugs against SARS-CoV-2 is largely centered on this spike protein, which binds to ACE2 expressed on human cells, as part of the process by which the virus gains entry into the cell. Before the SARS-CoV-2 spike protein can interact with ACE2, it changes shape to expose its receptor-binding domain (RBD), the part of the protein that interacts with ACE2.
Like many viral proteins, the SARS-CoV-2 spike protein has a thick coat of glycans on its surface. These glycans, which are attached at specific sites, help to shield the viral proteins from the host immune system. “Similar to many other viral fusion proteins, the SARS-CoV-2 spike utilizes a glycan shield to thwart the host immune response,” the scientists noted. For their newly reported work, the researchers investigated whether certain glycans in the SARS-CoV-2 spike protein might also be active players in the process leading to infection.
To look at this in more detail, the researchers used structural and glycomic data to build molecular dynamics simulations of the SARS-CoV-2 spike protein embedded in the viral membrane. “… we built a full-length model of the glycosylated SARS-CoV-2 S protein, both in the open and closed states, augmenting the available structural and biological data,” they noted. The computer models, which presented a detailed snapshot of every atom in the spike glycoprotein, revealed that N-glycans linked to the spike protein at certain sites (N165 and N234) helped to stabilize the shape change that exposes the RBD, and so could help to promote infection.
“We reveal an essential structural role of N-glycans at sites N165 and N234 in modulating the conformational dynamics of the spike’s receptor-binding domain (RBD), which is responsible for ACE2 recognition,” the authors stated. “This finding is corroborated by biolayer interferometry experiments, which show that deletion of these glycans through N165A and N234A mutations significantly reduces binding to ACE2 as a result of the RBD conformational shift toward the ‘down’ state.”
The scientists suggested that their findings could lay the foundation for new strategies to fight the SARS-CoV-2 pandemic. “Overall, this work provides an atomic-level perspective on the SARS-CoV-2 S protein, highlighting the importance of glycans not only as shielding devices for immune evasion but also as essential structural elements for virus infectivity,” they concluded. “These insights lay the foundations for a possible strategy to modulate the RBD conformational plasticity and virus infectivity, which could be harnessed in the development of therapeutics aimed at fighting the pandemic threat.”













