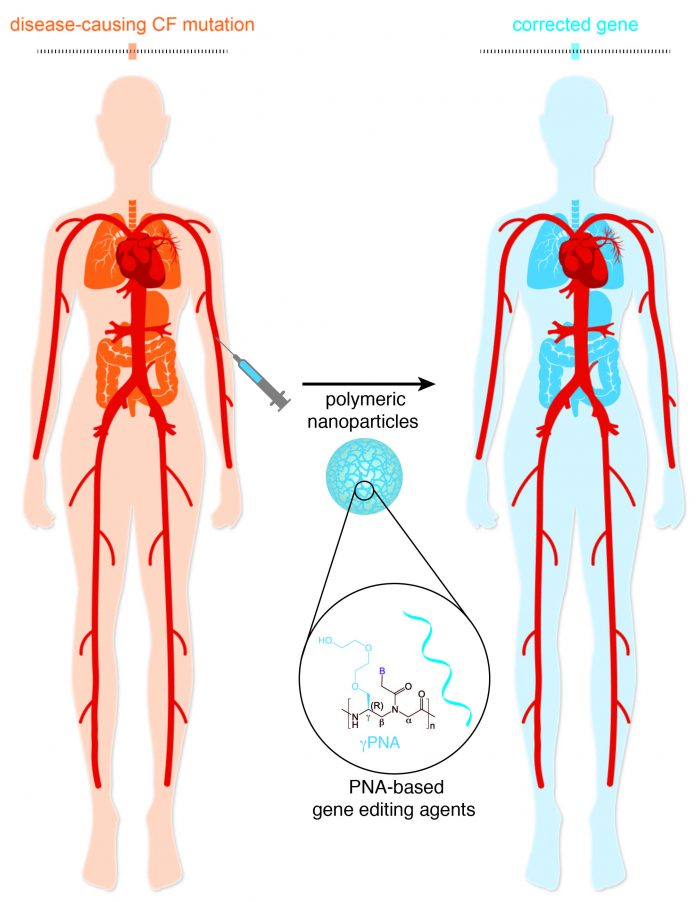
Gene editing technology that deploys a peptide nucleic acid (PNA) within a biocompatible polymer nanoparticle can correct a common mutation that causes cystic fibrosis (CF), a study in mice has revealed.
A single injection partially restored function of the F508del mutation in the CF transmembrane conductance regulator (CFTR) protein, which is the most common cause of the genetic disease.
CF symptoms reduced in the lungs and gastrointestinal (GI) tracts of mice and although response declined over time this was reversed with subsequent treatment, the researchers report in the Proceedings of the National Academy of Sciences.
Their results suggest PNA nanoparticles could be used to deliver treatment to the many areas impacted by the disease, which include not only in the respiratory system but also organs such as the pancreas and those of the digestive system.
“This work is an early proof-of-concept step toward developing a systemic treatment for cystic fibrosis that can be administered by simple intravenous injection,” senior study author Peter Glazer, from the Yale School of Medicine in New Haven, Connecticut, USA, told Inside Precision Medicine.
“The long-term goal is to ameliorate the multiple tissues in the body affected by the disease, such as the lung and GI tract.”
CF is caused by mutations in the gene producing the CFTR protein, which regulates the flow of water and salt components into and out of cells.
While the disease is best known for causing sticky mucus in the lungs that can clog airways and trap germs, it also affects multiple other organs and affects fertility.
Modulator therapies have shown great promise in mitigating the impact of the F508del mutation through increasing CFTR protein transport to the cell membrane and improving channel function, but treatment needs to be continuous, is expensive, and its impact in alleviating GI issues is unclear.
By contrast, gene editing approaches offers the potential for a one-time cure applicable to all CF mutations, including the one in 10 patients with rare variants who are not candidates for modulator therapies.
To investigate further, the researchers developed an approach to gene editing that stimulates DNA repair by binding a peptide nucleic acid to genomic DNA at the mutation site to create a PNA/DNA/PNA triplex structure.
They confirmed phenotypic and genotypic modification in vitro in primary nasal epithelial cells from F508del mice grown in a physiologically relevant air-liquid interface culture model.
In vivo treatment in mice homozygous for the F508del mutation resulted in partial gain of the CFTR function in epithelia, with mutation correction both in the airway and GI tissues and no off-target effects above background mutation/read error rates.
While the impact of treatment declined over time, this was reversed with additional treatment rounds.
“This is the first study to show that with a single intravenous administration of gene editing reagents multiple organs affected by CF can regain partial function of CFTR,” corresponding author Marie Egan, director of the Yale CF Center, told Inside Precision Medicine.
“This gene editing approach was tested in CF mice. If this treatment is optimized, the hope is that in the future this approach may be used to treat humans.”













