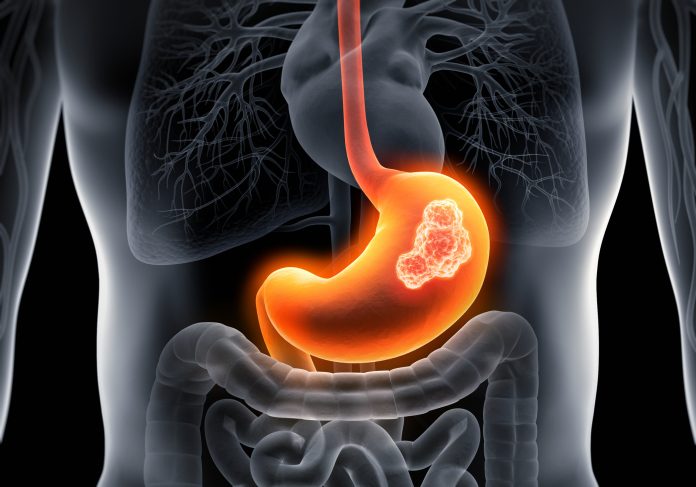
A new artificial intelligence tool predicts esophageal adenocarcinoma (EAC) and gastric cardia adenocarcinoma (GCA), a form of stomach cancer, at least three years prior to a diagnosis. Both cancers are highly fatal, and rates have risen sharply over the past five decades.
Researchers from the Lieutenant Colonel Charles S. Kettles Veterans Affairs Center for Clinical Management Research developed the machine learning model from the electronic medical records of 10 million US veterans. VA records are unique in that they are automatically linked to cancer registry outcomes allowing the researchers to look backwards in veterans’ health records for information that could be used to predict cancer. Their analysis included previous diagnoses, laboratory value results, weight, prescription history, and more.
“We were able to identify individuals who developed adenocarcinoma of the esophagus or esophageal junction and used a form of machine learning to learn more about them,” explains Joel Rubenstein, MD, a research scientist at the Kettles VA Center and professor of internal medicine at Michigan Medicine, who named the model the Kettles Esophageal and Cardia Adenocarcinoma predictioN tool, or K-ECAN.
The team accessed the Veterans Health Administration (VHA) Corporate Data Warehouse to identify veterans diagnosed with EAC (8,430) or GCA (2,965) over a 13-year period and compared them to 10,256,887 controls. The cancer cohort was split in half. One half was used to develop the K-ECAN model for predicting cancer, another quarter was used to tune the model, with the final quarter validating the results. “We found that the model predicts which individuals would develop these cancers at least three years before they did,” Rubenstein says. The model was more accurate than published guidelines in predicting cancer and more accurate than other tools that are already available that have been previously validated. Their findings were published in Gastroenterology.
The greatest identified risk factor was age, but others were found to be associated with increased cancer risk including Barrett’s esophagus, a precancerous condition, and gastroesophageal reflux disease (GERD). However, the model revealed other somewhat unexpected factors including slightly elevated hematocrit, low HDL/elevated LDL, lower blood serum bicarbonate levels, and greater white blood cell counts.
“All of the screening guidelines for esophageal cancer now rely on GERD symptoms—heartburn and reflux—to identify people who should get screening,” says Rubenstein. And while GERD is associated with the cancer, it wasn’t particularly important in terms of the amount of information provided to the model. Most people with GERD symptoms will never develop esophageal adenocarcinoma and gastric cardia adenocarcinoma. In addition, roughly half of the patients with this form of cancer never experienced prior GERD symptoms at all. “This makes K-ECAN particularly useful because it can identify people who are at elevated risk, regardless of whether they have GERD symptoms or not,” adds Rubenstein.
While current guidelines already consider screening in high-risk patients, Rubenstein notes that many providers are still unfamiliar with this recommendation and that fewer than 20% of people who have developed the cancer have had prior screening.
“We envision this tool being integrated seamlessly in the electronic health record to notify providers of their patients’ elevated risk,” Rubenstein explains. Providers would receive automated notification alerts regarding which patients are at an increased risk of developing ECA and GCA. They could then consider screening when an individual is due for a colonoscopy or when refilling acid-reducing medication as colonoscopy and upper endoscopy can be performed at the same time.
Currently, Rubenstein’s team is piloting the tool at the Kettles VA facility.