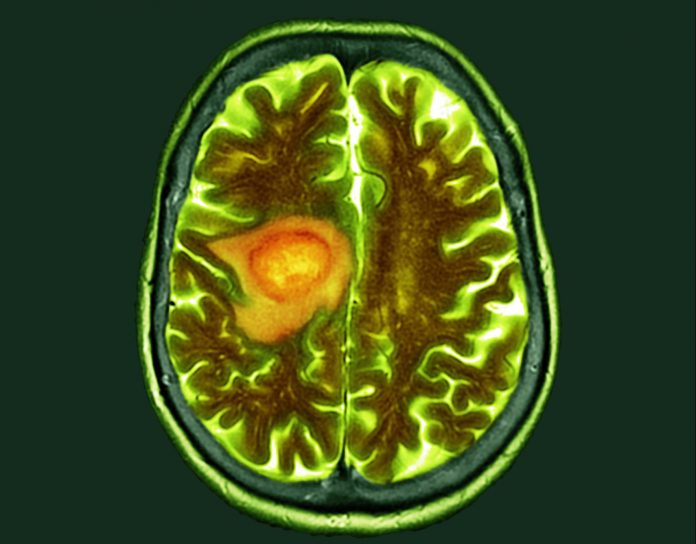
Early-stage research led by Brigham and Women’s Hospital shows that an engineered virus can significantly increase survival in patients with recurring glioblastoma brain tumors who have limited or no other treatment options.
The engineered virus—CAN-3110—was created by U.S. biotech Candel Therapeutics, which has a focus on creating novel immunotherapies to treat cancer. It is an engineered herpes simplex virus designed to target cancer cells by making them more visible to the immune system.
The viral neurovirulence ICP34.5 gene is present in CAN-3110, which is linked to the neural filament protein nestin. Nestin is present at high levels in glioblastoma and other similar tumors, but not in healthy adult brain tissue, making it a useful target.
“Recurrent high-grade gliomas including recurrent glioblastoma are characterized by rapid neurological morbidity and survival of less than ten months,” write E. Antonio Chiocca, a professor at Harvard Medical School and neurosurgeon at Brigham and Women’s Hospital, and colleagues in a Nature paper describing the Phase I study.
“Although much is known of the genetics, cellular composition and evolution of high-grade gliomas and glioblastoma, this has not translated into successful therapies.”
It is thought that the many trial failures linked to this kind of brain cancer is due, at least in part, to the extremely immune suppressive environment of these types of tumors.
In this study, 41 patients with recurrent glioblastoma, which typically has survival of six to nine months, were enrolled in the trial and injected with CAN-3110.
Overall, the treatment increased median overall survival to 11.6 months in trial participants. Notably, this went up to a median of 14.2 months in those with pre-existing herpes simplex antibodies and was only 7.8 months in those without herpes specific antibodies.
Two patients had seizures needing hospitalization that were thought to be related to CAN-3110, but no dose-limiting toxicities or adverse events linked to the presence of the viral neurovirulence gene ICP34.5 were observed.
“Importantly, approximately 30% of seronegative patients at baseline seroconverted after CAN-3110 treatment. Those patients also exhibited increased survival, suggesting the possibility of leveraging this mechanism and further improving survival upon multiple injections,” said Chiocca in a press statement.
The company now plans to further validate the data and test for safety with a view to progressing CAN-3110 towards expanded testing and eventual market approval for treatment of glioblastoma.
Candel is led by Paul Peter Tak, previously a senior VP and Chief Immunology Officer at GSK, as well as Professor of Medicine and Chair of the Department of Clinical Immunology and Rheumatology at the Amsterdam University Medical Center. It’s most advanced therapeutic candidate is an engineered adenovirus (CAN-2409), which is currently in late stage trials for treating prostate cancer.