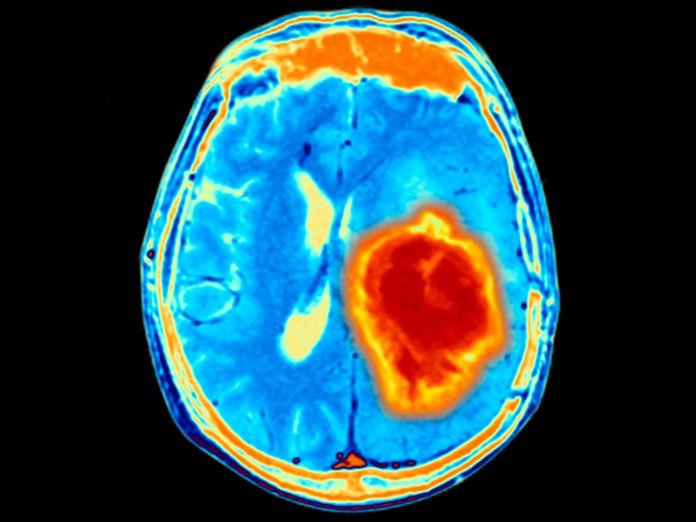
A new report in Trends in Cancer analyzes the prospects of tissue-agnostic therapeutics in patients with primary brain tumors (PBTs). The paper was written by researchers from Miami Cancer Institute, part of Baptist Health South Florida. In it, the researchers discuss data from clinical trials of tissue-agnostic targets for PBTs and describe additional tissue-agnostic targets that may hold promise for benefiting patients with PBTs.
The researchers note that valid tissue-agnostic targets for primary brain tumors, include BRAF V600E mutations, neurotrophic tyrosine receptor kinase fusions, high tumor mutational burden, and microsatellite instability (MSI), or mismatch repair deficiency. Future targets for tissue-agnostic approvals where utility in primary brain tumors remains to be demonstrated include FGFR, ERBB2/HER2, KRAS G12C, and TP53Y220C.
PBTs are tough to treat partly because of the blood brain barrier, which envelops the brain and limits drugs from reaching tumors. Also, brain tumors tend to be highly immunosuppressive, and as a result immunotherapies do not work well on them. These tumors also usually have multiple pathways driving them.
One of the key developments in oncology of the last couple decades is better understanding of the genetic markers driving tumor growth and spread. This, in turn, has allowed the design of therapies that can specifically target the cancer cells while sparing the normal cells. Mostly these were targeted to specific cancers. But, there’s a new sheriff in town—drugs that target tumors in any part of the body, as long as they have certain markers.
“Tumor agnostic approvals have changed how we view cancer,” first author Manmeet S. Ahluwalia, MD, tells Inside Precision Medicine. Ahluwalia is chief of medical oncology and chief scientific officer at Miami Cancer Institute.
He explains that a better understanding of the tumor biology in the last decade has also shown there can be similar genetic alterations that may be driving a lung cancers that may also be driving a melanoma.
“Novel tissue-agnostic therapeutics targeting driver mutations in tumor cells have been recently approved by the FDA and other regulatory bodies across the world, driven by trials that have demonstrated their efficacy and safety across diverse tumor histology,” says Ahluwalia. “However, the relative rarity of primary brain tumors has limited their representation in early trials of tissue-agnostic medications.”
PBTs have substantial genetic, epigenetic, and immunological heterogeneity, which adds to the complexity of the tissue-agnostic therapies. Innate differences between various types of PBTs must be considered carefully because even within the same tumor, the authors note, multiple subtypes within the same spectrum can exist, adding to the histological heterogeneity.
“However, the limited number of patients with different PBTs in published studies hampers their widespread uptake,” said Ahluwalia. “Therefore, it is becoming increasingly crucial for individuals with primary brain tumors to undergo molecular profiling, enabling the maximization of therapeutic options based on individualized characteristics and for real-world analytics to capture this data. These tissue-agnostic approvals, as the new frontier of precision oncology, hold promise for improved treatment outcomes of gliomas.”
He said his team is building a new cadre of trials focused on extensive genetic profiling of a patient’s tumors to learn what are the genetic alterations driving someone’s particular cancer. Then they will match the patient with the right drug or right clinical trial to advance science, “fulfilling the premise of precision medicine to the highest level,” Ahluwalia says.